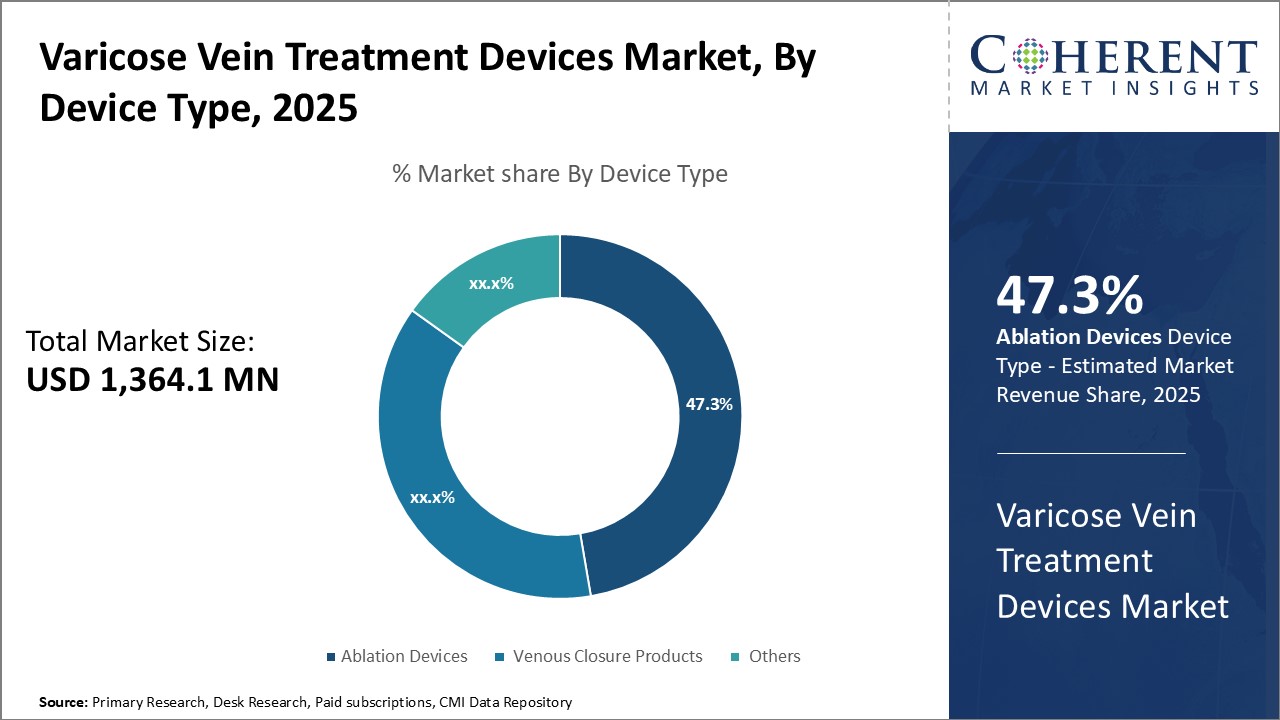
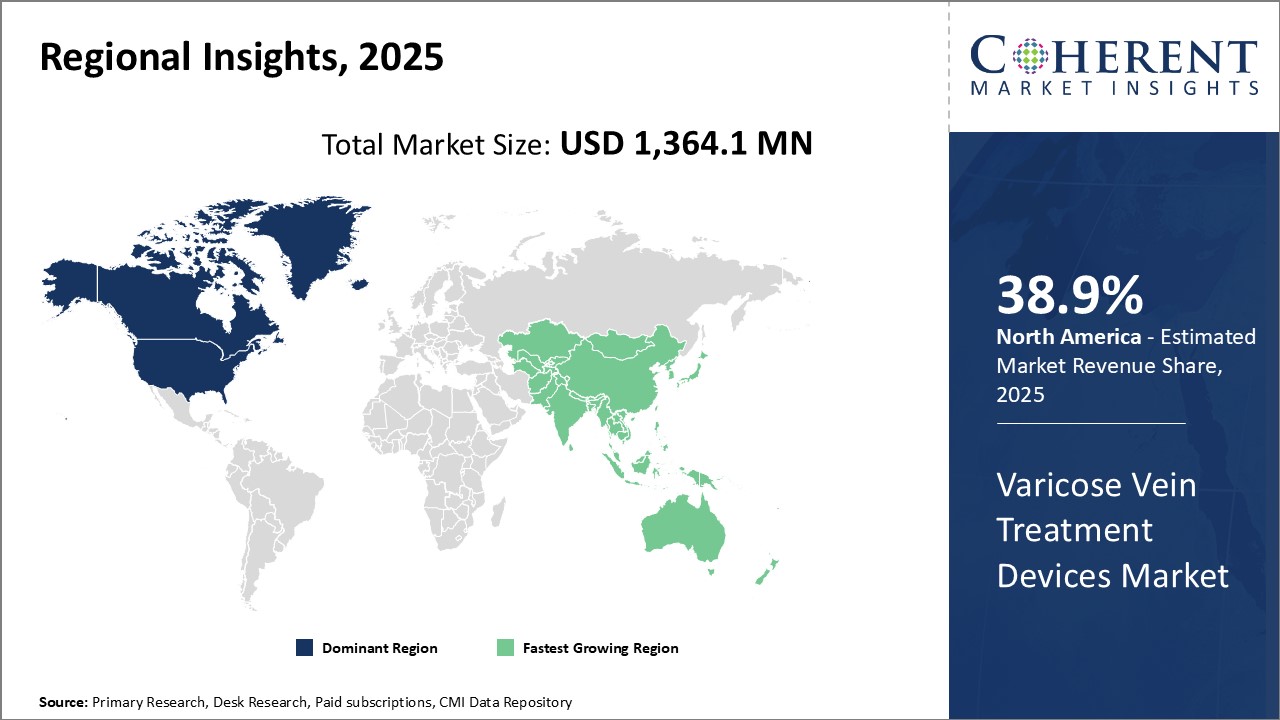
The varicose vein treatment devices market is estimated to be valued at USD 1,364.1 Mn in 2025 and is expected to reach USD 2,163.3 Mn by 2032, growing at a compound annual growth rate (CAGR) of 6.8% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The varicose vein treatment devices market is expected to witness positive growth over the forecast period. There is an increasing prevalence of varicose veins around the world owing to rising obesity levels and sedentary lifestyles. Advanced treatment procedures, such as endothermal ablation and ultrasound-guided foam sclerotherapy, are gaining popularity over traditional surgery due to benefits such as less pain, fewer complications, and faster recovery. Further, favorable reimbursements and increasing awareness about varicose vein treatments are also fueling the adoption of varicose vein treatment devices.
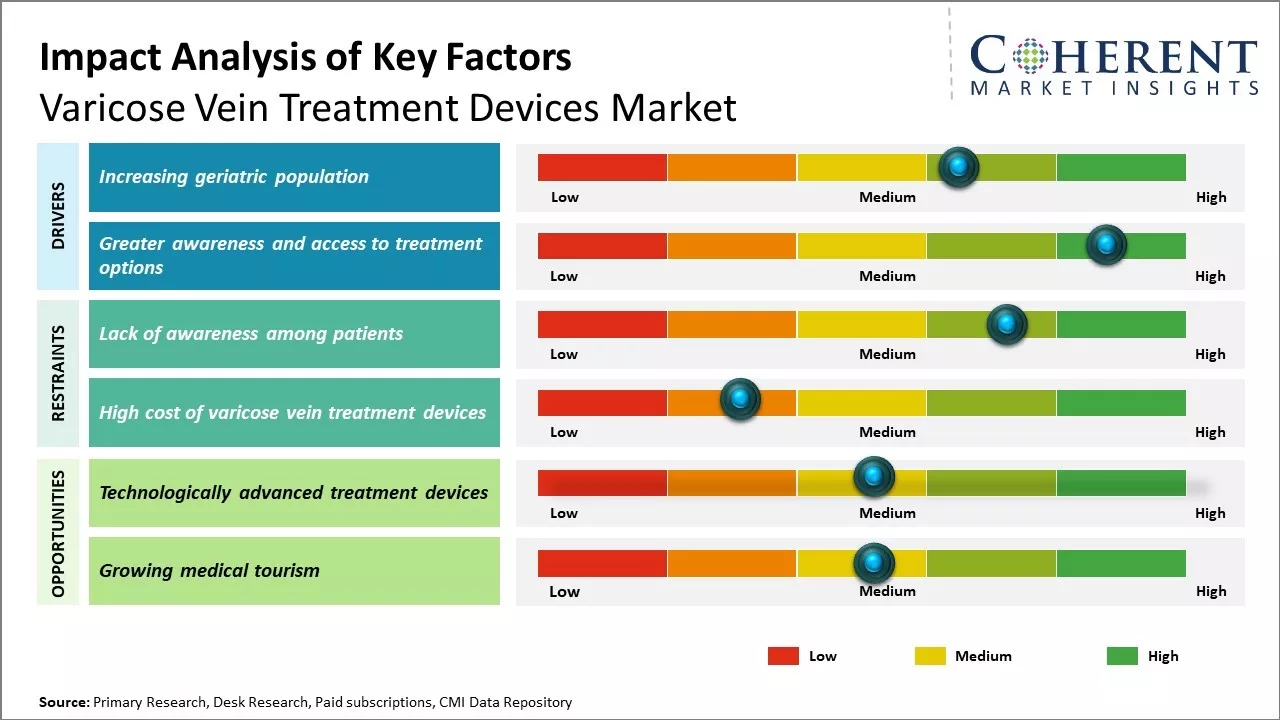
Increasing geriatric population
Increasing geriatric population is expected to drive market growth during the forecast period. Aging causes wear and tear on the valves in the veins that help control blood flow. Eventually, that wear causes the valves to get weaker, allowing for the blood to flow backwards. The pressure from the increased amount of blood within the vein build ups results in twisted, bulging veins known as varicose veins. For instance, according to data published in April 2021 on Canada.ca, a Canadian government website over 861,000 people aged 85 and older were counted in the Canada 2021 Census.
Get actionable strategies to beat competition: Download Free Sample
Greater awareness and access to treatment optionsIn the past, varicose veins were generally considered a cosmetic issue. This led to many people dismissing the condition or ignoring it. However, over the past decade there has been a significant increase in public health awareness campaigns around varicose veins. People are now more cognizant that varicose veins are not just a cosmetic issue but can also be symptomatic and cause issues such as leg pain, fatigue, swelling, skin discoloration or ulcers in severe cases. As such, the condition is perceived to require treatment rather than be ignored. Accompanying this rise in awareness, there have also been major advancements in varicose vein treatment technologies and techniques. Minimally invasive procedures such as endovenous laser ablation and radiofrequency ablation have largely replaced conventional surgeries as treatment options. These newer techniques offer advantages such as being on outpatient basis, minimal incisions, lesser pain, and faster recovery. Additionally, the availability of experienced phlebologists and vein treatment centers has expanded greatly.

To learn more about this report, Download Free Sample
Market Challenges: Lack of awareness among patientsOne of the major challenges for the varicose vein treatment devices market is the lack of awareness among patients about newer minimally invasive treatment options. Many patients still prefer traditional surgery which requires long recovery time and has potential complications. Additionally, the high cost of laser and radiofrequency varicose vein treatment devices puts them out of reach for many patients. Reimbursement issues and lack of coverage for newer treatment types in many countries also limit the adoption of modern devices.
Market Opportunities: Technologically advanced treatment devices
The ability of newer devices to treat varicose veins without scarring or long downtime makes them attractive to many. As these procedures become more routine and cost decreases with technological advancements, more patients and healthcare providers will opt for them. An aging population and rising obesity rates have also increased the incidence of varicose veins, thereby creating lucrative opportunities for market growth over the projected period.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
Insights, By Product Type: Technological Advancements Fuel the Popularity of Ablation DevicesThe product type segment includes ablation devices (radiofrequency ablation devices, laser ablation devices), venous closure products, and others. Ablation devices segment is anticipated to hold 47.3% of the market share in 2025. Ablation devices utilize various sources of energy like radiofrequency, laser, or heat to seal veins shut and reroute blood flow. Newer devices deliver more precise energy doses, can treat different vein sizes and lengths, and cause minimal damage to surrounding tissues. This has reduced risks of side effects like burns or nerve damage. Ablation treatments also allow for ambulatory procedures with faster recovery times compared to surgical techniques. Various innovations such as using ultrasound or thermal monitoring have enabled ablation to treat complex cases that were earlier considered only for surgery. This has expanded the eligible patient pool for these devices. Additionally, advanced ablation technologies provide long-lasting treatment outcomes equivalent to surgery while reducing costs for both patients and healthcare systems over time. As a result, ablation devices remain the preferred first-line treatment option for most varicose vein conditions.
Insights, By Treatment Type: Preference for Minimally Invasive Procedures Boosts Endovenous Ablation
The treatment type segment includes sclerotherapy, endovenous ablation, surgical ligation & stripping, and others. Endovenous ablation contributes the highest share of the varicose vein treatment devices market and is projected to hold 45.3% of the market share in 2025. Endovenous techniques deliver energy directly inside the malfunctioning vein through catheters or fibers to seal it shut. This eliminates the need for large incisions or general anesthesia typically required for surgical techniques. Endovenous ablation translates to less post-procedural pain, faster recovery times, and minimized scarring and risk of complications for patients. It also holds advantages for providers in terms of reduced procedure duration and higher treatment volumes within the same facility resources. While older methods caused post-procedure nerve pain or hyperpigmentation in some cases, current endovenous technologies minimize such side effects much more reliably. As patient awareness of minimally invasive options is rising continuously, more individuals are choosing endovenous ablation over surgical treatments. The popularity of this treatment approach is further facilitated by technicians being trained in performing it. Overall, the capacity of endovenous ablation to provide effective varicose vein relief through small access points contributes most to its popularity.
Insights, By End User: Convenience and Growing Popularity Drives Specialty Clinics Growth
The end user segment includes specialty clinics, hospitals, ambulatory surgical centers, and others. Specialty clinics contribute the highest share of the varicose vein treatment devices market and is projected to hold 38.7% of the market share in 2025. Varicose vein treatments require skill and experience to apply technologies appropriately and achieve optimal outcomes. Specialty vein clinics employ physicians who are trained and board-certified phlebologists/vascular surgeons adept at different modalities. They also maintain top equipment and support staff to handle a variety of cases. This caliber of expertise may not always be uniformly available across hospitals and primary care centers. Besides skilled treatment, specialty centers can also dedicate more resources to educating patients, providing follow up support, and developing personalized management plans. Their exclusive focus on vein care further assures patients about quality and safety standards. While hospitals may be preferred for complex cases, uncomplicated varicose vein conditions are more patronized by cost- and time-conscious individuals at specialty clinics of reputed experts. Overall, the centralized availability of hyper-specialized vein physicians forms the cornerstone driving the segmental edge of specialty clinics.
Need a Different Region or Segment? Download Free Sample
North America has established itself as the dominant player in the global varicose vein treatment devices market and is expected to hold 38.9% of the market share in 2025. In North America, Ablation Devices account for the largest share of the Varicose Vein Treatment Devices market by Product Type. Ablation Devices work by targeting veins with thermal energy sources like laser or radiofrequency to cauterize diseased veins. A major contributor to their market dominance is an increasing preference for minimally invasive procedures among physicians and patients in the region. Within Ablation Devices, endovenous radiofrequency ablation (RFA) systems have been particularly successful. RFA works by emitting radiofrequency energy through a catheter to heat and seal varicose veins. Leading RFA device manufacturers serving the North American market like AngioDynamics and VNUS Medical have seen steady growth based on the rising adoption of RFA in ambulatory surgical centers and physician offices.
Asia Pacific has emerged as the fastest growing regional market for varicose vein treatment devices. Countries like India, China, Japan, and South Korea are witnessing exponential growth opportunities. A key driver of the ablation devices segment has been the introduction of endovenous laser ablation (EVLA) and radiofrequency ablation (RFA) techniques. These modern ablation methods allow for effective treatment of varicose veins using small, thin catheters inserted into the leg instead of traditional surgery. This minimizes recovery time for patients and reduces risks like wound infections. Several clinical studies from public hospitals across Asia have demonstrated ablation techniques like EVLA and RFA to have high treatment success rates of over 95.5% for eliminating varicose veins.
Varicose Vein Treatment Devices Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1,364.1 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.8% | 2032 Value Projection: | USD 2,163.3 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
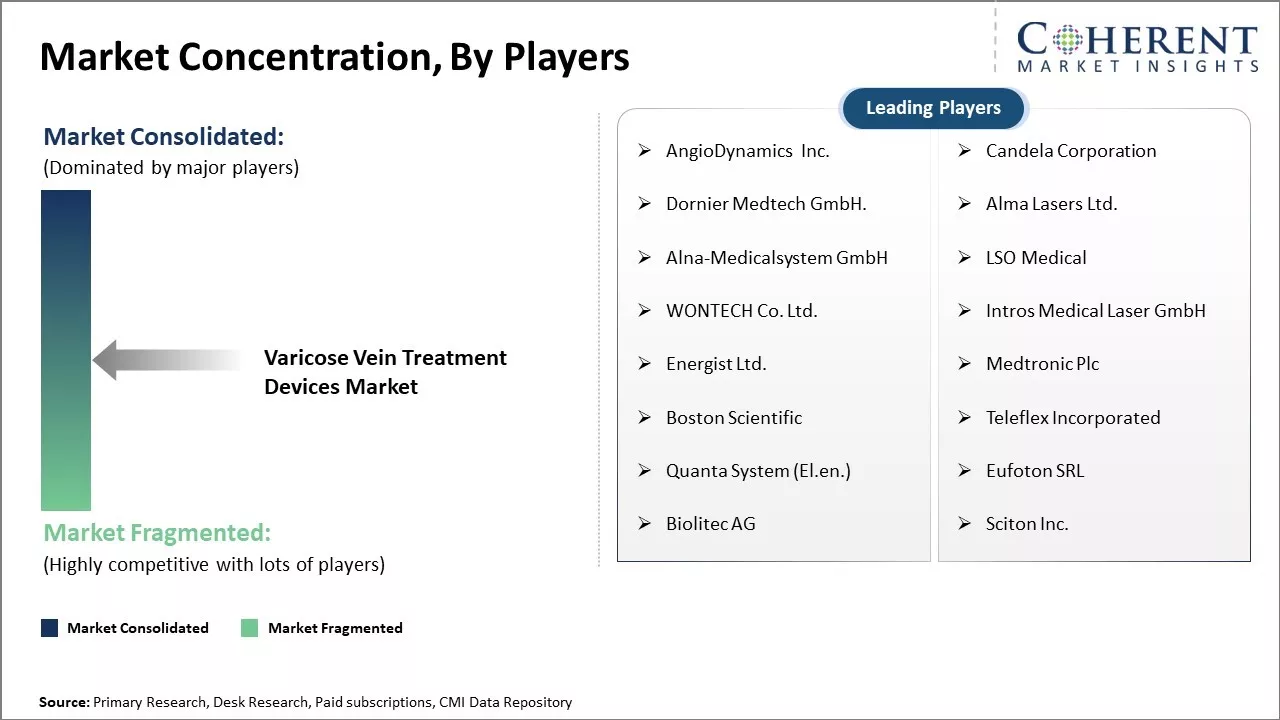
| Companies covered: |
AngioDynamics Inc., Candela Corporation, Dornier Medtech GmbH., Alma Lasers Ltd., Alna-Medicalsystem GmbH, LSO Medical, WONTECH Co. Ltd., Intros Medical Laser GmbH, Energist Ltd., Medtronic Plc, Boston Scientific, Teleflex Incorporated, Quanta System (El.en.), Eufoton SRL, Biolitec AG, Sciton Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients