U.S. Vaccine Market is estimated to be valued at USD 26.59 Bn in 2025 and is expected to reach USD 39.2 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 5.7% from 2025 to 2032.
Analysts’ Views on U.S. Vaccine Market:
The initiation of vaccine development through mRNA technology is increasing research and development (R&D) activities owing to the prevalence of various infectious diseases, such as Ebola, Zika, and influenza, among others. This trend has been fuelled due to the emergence of SARS-CoV-2, which is surging the requirement for effective vaccines to prevent morbidity and fatality, which has driven the market. As research activities to develop efficient vaccines were initiated, mRNA technology was leveraged to easily modify the sequencing of vaccines to enable their effectiveness against the mutations of the novel virus, which has driven market growth.
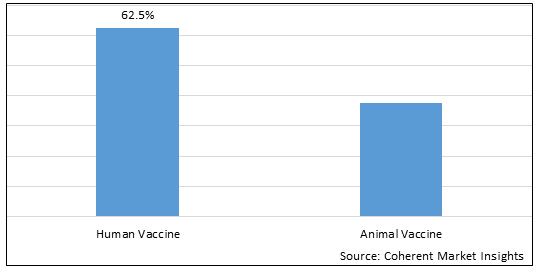
Figure 1. U.S. Vaccine Market Share (%), by Product Type, 2025
To learn more about this report, Download Free Sample
U.S. Vaccine Market – Driver
Emergence of COVID-19
The emergence of Covid-19 is expected to propel the growth of the U.S. vaccines market over the forecast period. For instance, according to an article published by World Health Organization in January 2020, there have been 2,833,552 confirmed cases of COVID-19 with 129,408 deaths in the U.S.
R&D initiatives for new vaccines
R&D initiatives for new vaccines by key market players is also expected to aid in the growth of the market. For instance, in March 2020, IQVIA is a multinational company serving the combined industries of health information technology and clinical research in U.S., and Quest Diagnostics is Clinical laboratory, through its joint venture Q2 Solutions, collaborated with the University of Texas Medical Branch to develop a novel assay for COVID-19 tests for the rapid development of a Coronavirus vaccine.
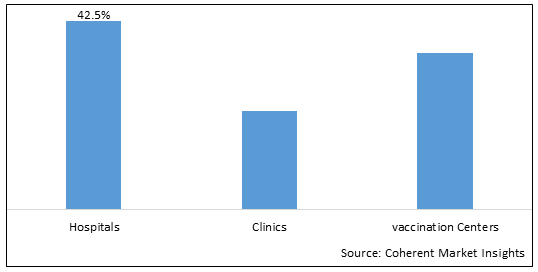
Figure 2. . U.S. Vaccine Market Share (%), by End User, 2025
To learn more about this report, Download Free Sample
U.S. Vaccine Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, the U.A.E., Egypt, and others are facing problems with the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a positive impact on the U.S. Vaccine market. For instance, on January 19, 2023, according to the World Health Organization, per year around, 35,172 human deaths and 21,476 human deaths occur due to rabies in North America. The cases of patients suffering from polio in Asia Pacific are reducing because of increasing awareness regarding the prevention of polio and rabies among the population and free immunization camps, which are organized by the government for immunizing the population against polio and rabies. Thus, North America is also projected to spur the U.S. vaccines market growth over the forecast period.
U.S. Vaccine Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 26.59 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.7% | 2032 Value Projection: | USD 39.2 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Major players operating in the U.S. Vaccine Market include Sanofi Pasteur, GlaxoSmithKline Plc, Merck & Company Incorporated, Pfizer Incorporated, AstraZeneca plc, Boehringer Ingelheim, Bharat Biotech., BIO-MED, Serum Institute of India Pvt. Ltd., Kedrion Biopharma Inc. GlaxoSmithKline plc., Bilthoven Biologicals, Cadila Healthcare Limited, Sanofi Pasteur India Pvt Ltd, Chiron Behring Vaccines Private Ltd, Zoetis Inc., Elanco., Boehringer Ingelheim International GmbH, Indian Immunologicals Ltd, Wyeth pharmaceuticals, Berna Biotech Ltd., Novartis Vaccines Ltd, and Medimmune LLC. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
U.S. Vaccine Market Segmentation:
The U.S. Vaccine market report is segmented into By Product Type and By End User,
By Product Type, the market is segmented into human vaccine (pediatric Vaccines, Adult & Adolescent Vaccines, Other Human Vaccines) and Animal Vaccine (Livestock Vaccines, Companion Animal Vaccines, Other Animal Vaccines). Out of which, the Human Vaccine (Pediatric Vaccines, Adult & Adolescent Vaccines, Other Human Vaccines) segment is expected to hold a dominant position in the U.S. Vaccine market during the forecast period and this is attributed to the increasing awareness of vaccines.
By End User, the market is segmented into Hospitals, Clinics, and Vaccination Centers. Out of which, the Hospitals segment is expected to hold a dominant position in the U.S. Vaccine market during the forecast period and this is attributed to hospitals taking the initiative for vaccines camp.
U.S. Vaccine Market: Key Developments
In April 2022, Cadila Pharmaceuticals an multinational pharmaceutical company based in India, launched the world’s first three-dose anti-rabies vaccine. Its recombinant nano particle-based rabies G protein vaccine.
In September 2021, Serum Institute of India Pvt. Ltd Pharmaceutical company in India had started supplying Inactivated Polio Vaccine (IPV) to the Union government for the Universal Immunization Programme. Union health ministry had placed a purchase order with the Serum Institute of India for 54 lakh doses of IPV vaccine.
In June 2022, Bharat Biotech ltd. is a biotechnology company, introduced a new variant of INDIRAB is prepared using the strain obtained from the Centers for Disease Control and Prevention, USA. A cell culture-derived vaccine, it is produced in Vero Cells, inactivated and chromatographically purified.
In 2021, GlaxoSmithKline plc announced that they submitted a Biologics License Application (BLA) to the USFDA for its experimental vaccination PRIORIX. Over 100 nations have recently granted licenses for the vaccine, initially registered in Germany.
In 2020, GlaxoSmithKline plc and Medicago Inc., two of Mitsubishi Tanabe Pharma Corporation's subsidiary companies, announced their collaboration. They established a partnership to create a plant-based virus-like particle (VLP) vaccine for COVID-19.
U.S. Vaccine Market: Key Trends
Major players in the market are focused on adopting partnership and collaboration strategies to expand their product portfolio
Major players in the market are focused on adopting partnership and collaboration strategies to expand their product portfolio is expected to propel the growth of U.S. vaccine market over the forecast period. For instance, in April 2020, Sanofi Pasteur, supplies healthcare professionals with a range of vaccines, collaborated with GSK, a pharmaceutical and biotechnology company with global headquarters in London, to develop an adjuvanted vaccine for COVID-19.
Stringent regulations that promote vaccination
Stringent regulations that promote vaccination is expected to propel the growth of U.S. Vaccine market over the forecast period. For instance, in January 2020, The Government of New Jersey, U.S., mandated college students to be vaccinated against bacterial meningitis as a condition for attending an institution.
U.S. Vaccine Market: Restraint
High cost of refrigeration of vaccines
High cost of refrigeration of vaccines is expected to hinder the growth of the market. For vaccines, refrigeration is mandatory to prevent alteration of their chemical structure mainly due to contact with water and humidity resulting in ineffective medications. Maintaining this cold chain from production until they are used in treatment is a costly process accounting for about 80% of the price of vaccinations. For instance, The vaccine, developed by Pfizer and the German firm BioNTech, seems to provide 90% immunity according to early data released. The vaccine has to be stored at -70 degrees Celsius. freezers don’t get that cold, making distribution of this vaccine refrigeration which cost US$10,000 to US$15,000 each.
Market Players are taking initiative to launch cost-effective products.
U.S. Vaccine Market - Key Players
Major players operating in the U.S. Vaccine market include Sanofi Pasteur, GlaxoSmithKline Plc, Merck & Company Incorporated, Pfizer Incorporated, AstraZeneca plc, Boehringer Ingelheim, Bharat Biotech., BIO-MED, Serum Institute of India Pvt. Ltd., Kedrion Biopharma Inc. GlaxoSmithKline plc., Bilthoven Biologicals, Cadila Healthcare Limited, Sanofi Pasteur India Pvt Ltd, Chiron Behring Vaccines Private Ltd, Zoetis Inc., Elanco., Boehringer Ingelheim International GmbH, Indian Immunologicals Ltd, Wyeth pharmaceuticals, Berna Biotech Ltd., Novartis Vaccines Ltd, and Medimmune LLC.
*Definition: A vaccine is a biological preparation that provides active acquired immunity to a particular infectious disease. Inactivated, attenuated, toxoid, subunit, conjugate, heterotypic, experimental, and valence are the types of vaccines.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients