The U.S. sterile injectables market is estimated to be valued at USD 170.71 Bn in 2025 and is expected to reach USD 328.67 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 9.8% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
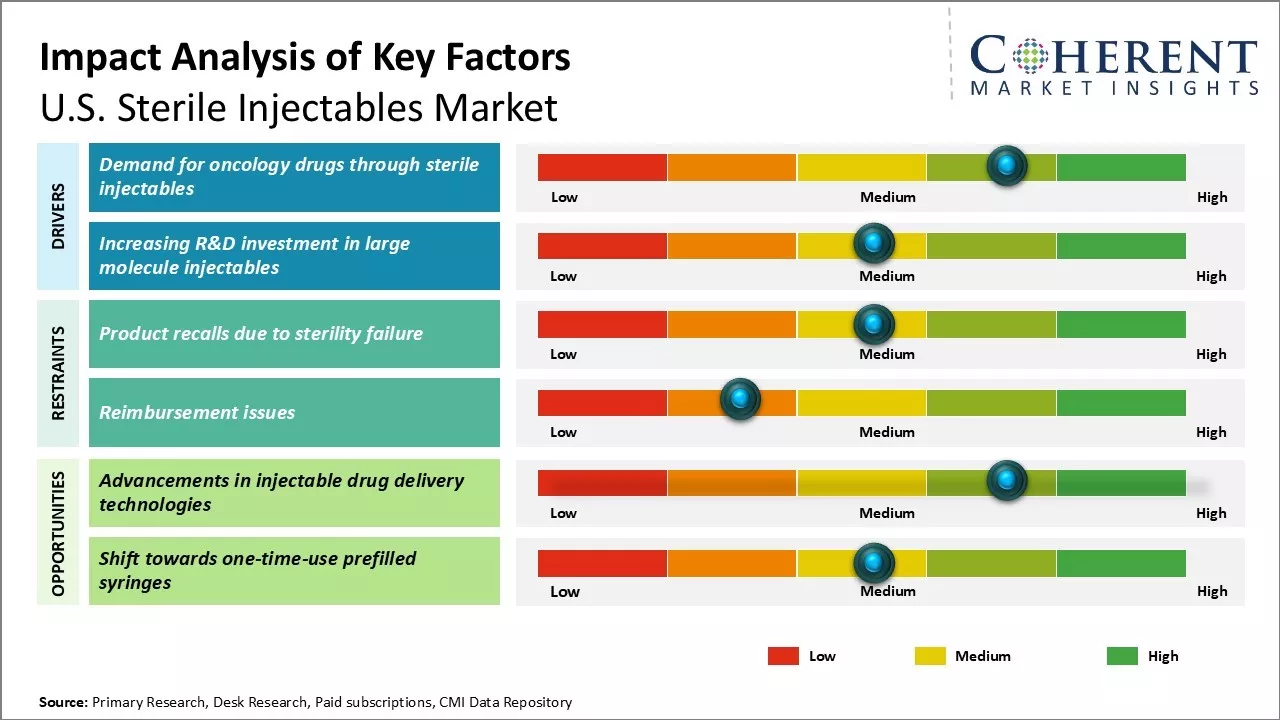
The U.S. sterile injectables industry is experiencing robust growth, driven by the rising prevalence of chronic diseases such as cancer and diabetes. The increasing reliance on injectable therapies for effective treatment options enhances market growth. However, challenges include high development costs and regulatory hurdles that can delay product approvals. Additionally, market players face competition from alternative drug delivery methods, which may restrict the market growth. Despite these restraints, advancements in biotechnology and personalized medicine are expected to provide new opportunities for innovation and expansion within the sterile injectables sector.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
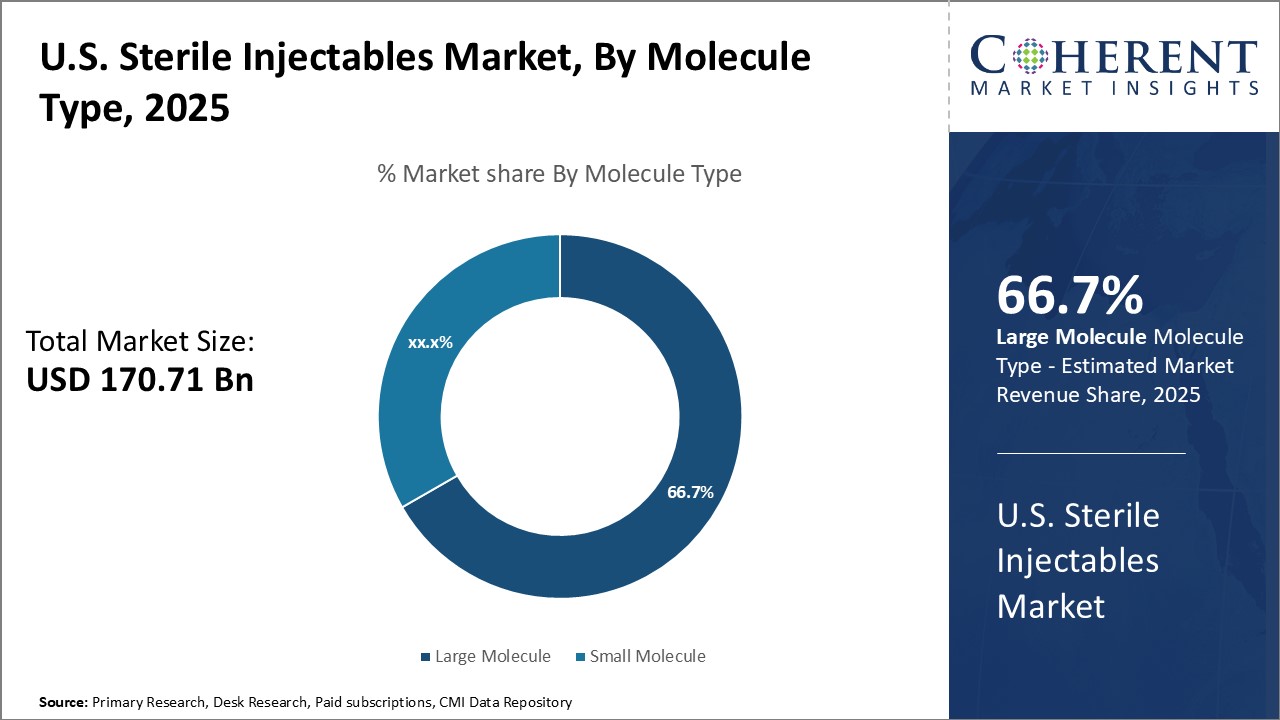
Insights by Molecule Type: The rise of biologics is driving the large molecule segment growth
In terms of molecule type, large molecule segment is expected to contribute the highest market share of 66.7% in 2025, driven by the rapid growth of biologics. Monoclonal antibodies are increasingly preferred for treating chronic diseases due to their high specificity and efficacy. However, challenges such as complex manufacturing processes and high costs may hinder market expansion.
Insights by Drug Type: Monoclonal antibody’s supremacy in cancer treatment drives the segment growth
In terms of drug type, monoclonal antibody (mAbs) segment is expected to contribute the highest market share of 48.6% in 2024, driven by their pivotal role in treating various cancers and chronic inflammatory diseases. mAbs have become essential for treating various cancers and chronic inflammatory diseases. Continuous innovation in antibody engineering is expanding treatment indications, ensuring that monoclonal antibodies remain dominant in the U.S. sterile injectables industry.
Insights by Therapeutic Application: Increasing Prevalence of Cancer Drives Segment Growth
In terms of therapeutic application, cancer segment is expected to contribute the highest market share of 53.9% in 2025. Cancer prevalence is increasing significantly driven by aging populations and changing lifestyles globally. It has become one of the leading causes of mortality, elevating the demand for advanced oncology treatment options.
Get actionable strategies to beat competition: Download Free Sample
Top Strategies Followed by U.S. Sterile Injectables Market Players
Emerging Startups in the U.S. Sterile Injectables Market
Key Takeaways from Analyst
U.S. Sterile Injectables Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 170.71 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.8% | 2032 Value Projection: | USD 328.67 Bn |
| Segments covered: |
|
||
| Companies covered: |
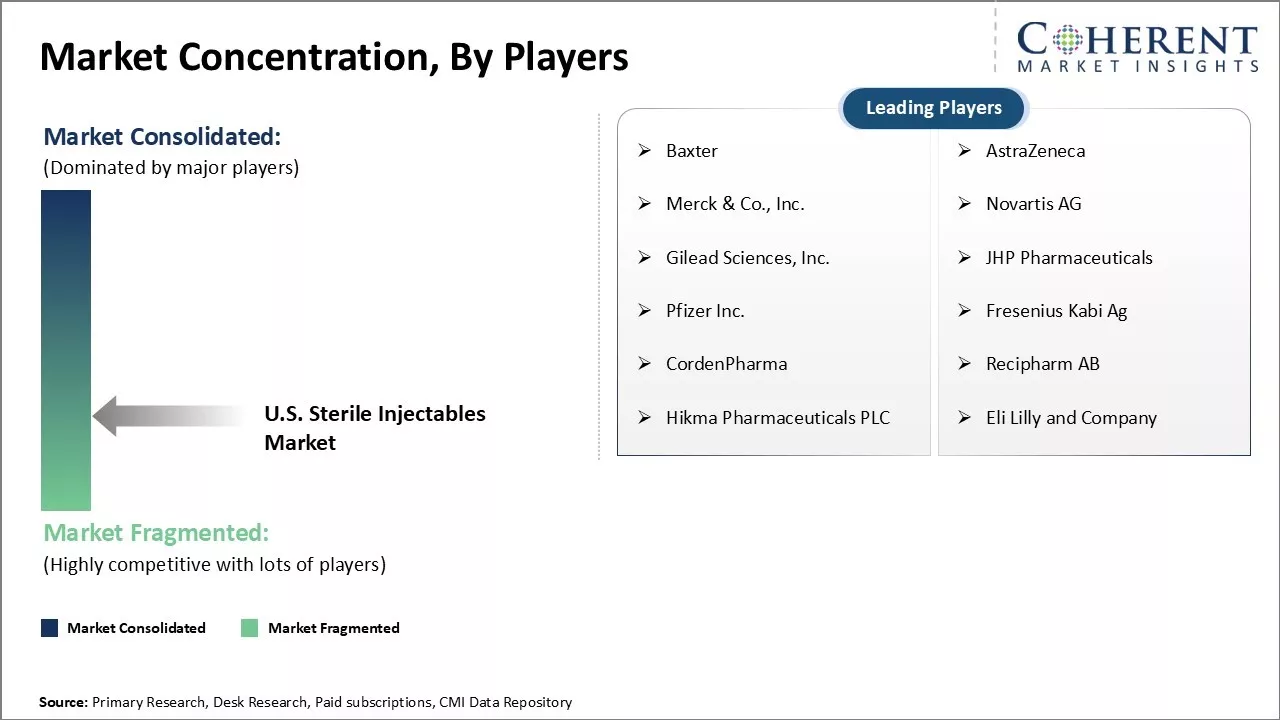
Baxter, AstraZeneca, Merck & Co., Inc., Novartis AG, Gilead Sciences, Inc., JHP Pharmaceuticals, Pfizer Inc., Fresenius Kabi Ag, CordenPharma, Recipharm AB, Hikma Pharmaceuticals PLC, and Eli Lilly and Company |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Driver - Demand for oncology drugs through sterile injectables
The rising prevalence of cancer is driving a significant demand for effective treatment options, as it remains a leading cause of mortality worldwide, impacting millions each year. Scientific studies project that new cancer cases will continue to increase due to lifestyle changes and an aging population. This trend poses a major challenge for healthcare systems, necessitating the development of innovative therapies to address the growing complexity of cancer care and improve patient outcomes.
Market Challenge - Product recalls due to sterility failure
The U.S. sterile injectables industry is grappling with significant challenges due to product recalls stemming from sterility failures. Even minor sterility issues can lead to severe health risks, prompting large-scale recalls that burden manufacturers financially and damage brand reputations. Stricter regulatory norms will require increased investment in advanced manufacturing and quality systems, potentially straining profit margins for suppliers.
Market Opportunity - Advancements in injectable drug delivery technologies
The U.S. sterile injectables market is poised for growth due to advancements in drug delivery technologies. Innovations like pre-filled syringes, auto-injectors, and microencapsulation are improving patient experiences and adherence, particularly for chronic conditions. Additionally, alternative administration methods, such as 3D-printed patches, are expanding treatment options, enhancing the market's potential for accelerated drug development.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients