U.S. Safety Needles Market is estimated to be valued at USD 3.1 Bn in 2025 and is expected to reach USD 4.3 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 4.8% from 2025 to 2032. Increasing product launches by key market players is expected to drive the market growth over the forecast period. Moreover, increasing product approvals and inorganic strategies such as collaborations and partnerships are expected to drive the U.S. safety eedles market growth.
Analysts’ Views on U.S. Safety Needles Market:
Rising number of patients with diabetes and cancer, among other diseases that need safety needles for the treatment and diagnosis of disease is expected to create opportunity for various manufacturers to offer different types of safety syrinegs such as prefilled syringe needle, A.V. fistula needle, and cannula needle among others.
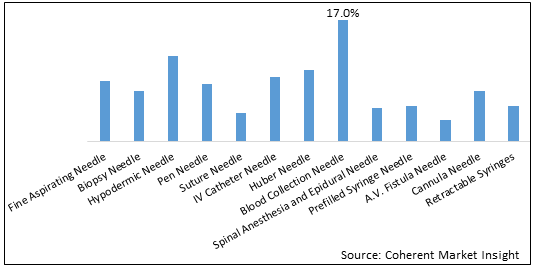
Figure 1. U.S. Safety Needles Market Share (%), By Product Type, 2025
To learn more about this report, Download Free Sample
U.S. Safety Needles Market- Drivers
U.S. Safety Needles Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization (WHO) declared it a public health emergency on January 30, 2020.
COVID-19 has affected the economy in three main ways: by directly affecting the production and demand for medical devices, by creating disruptions in modality, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, UAE, Egypt, and others faced problems regardingthe transportation of medical devices from one place to another.
Quarantine, traveling constraints, and social distancing measures are likely to lead to a steep decline in business and consumer spending. Furthermore, healthcare providers faced challenges such as lack of additional manpower, equipment, consumables, and other resources that were required to ensure safety in hospitals and provide treatment to patients with other diseases. Though the impact of COVID-19 on the U.S. safety needle market was positive as government intiatives for the manufacturing of safety needles used in the syringes were increased during the pandemic. For instance, in November 2020, ApiJect Systems, Corp., a U.S.-based healthcare company, announced that it had been granted a US$ 590 Million loan by the U.S. International Development Finance Corporation (DFC). A high-quality needle hub facility wasbuilt with the help of the DFC loan and a sizable amount of private funding that reducedApiJect's dependency on international supply chains significantly.
U.S. Safety Needles Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3.1 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.8% | 2032 Value Projection: | USD 4.3 Bn |
| Segments covered: |
|
||
| Companies covered: |
Medtronic Plc., Becton, Dickinson and Company, Boston Scientific Corporation, Smith Medical, Abbott Laboratories, Argon Medical Devices Inc., Novo Nordisk A/S, Terumo Corporation, Eli Lilly and Company, Nipro Corporation, B.Braun Melsungen AG, Revolutions Medical Corporation, Retractable Technologies, Inc, SOL-MILLENNUM, UltiMed, Inc., Axel Bio Corporation, Inc., Cardinal Health, Inc., Vygon S.A., and Gerresheimer AG. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
U.S. Safety Needles Market Segmentation:
The U.S. safety needles market is segmented into product type, application, end user, and distribution channel.
U.S. Safety Needles Market Cross Sectional Analysis:
In product type segment, blood collection needle segment is expected to dominate in U.S. over forecast period as key market players such as BD, Medline, AdvaCare Pharma USA LLC, Nipro Medical among others are offering the blood collection needles
U.S. Safety Needles Market: Key Developments
U.S. Safety Needles Market: Key Trends
U.S. Safety Needles Market: Restraint
U.S. Safety Needles Market: Key Players
Major players operating in the U.S. safety needles market include Medtronic Plc., Becton, Dickinson and Company, Boston Scientific Corporation, Smith Medical, Abbott Laboratories, Argon Medical Devices Inc., Novo Nordisk A/S, Terumo Corporation, Eli Lilly and Company, Nipro Corporation, B.Braun Melsungen AG, Revolutions Medical Corporation, Retractable Technologies, Inc, SOL-MILLENNUM, UltiMed, Inc., Axel Bio Corporation, Inc., Cardinal Health, Inc., Vygon S.A., and Gerresheimer AG.
Definition: When the safety needle is activated, a strong shield surrounds the entire needle. Once the safety shield has been locked into place, the gadget cannot be deactivated due to the unique integrated locking mechanism thatmay be actuated with a thumb, finger, or flat surface. It frequently pairs with luer lock or luer connection devices.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients