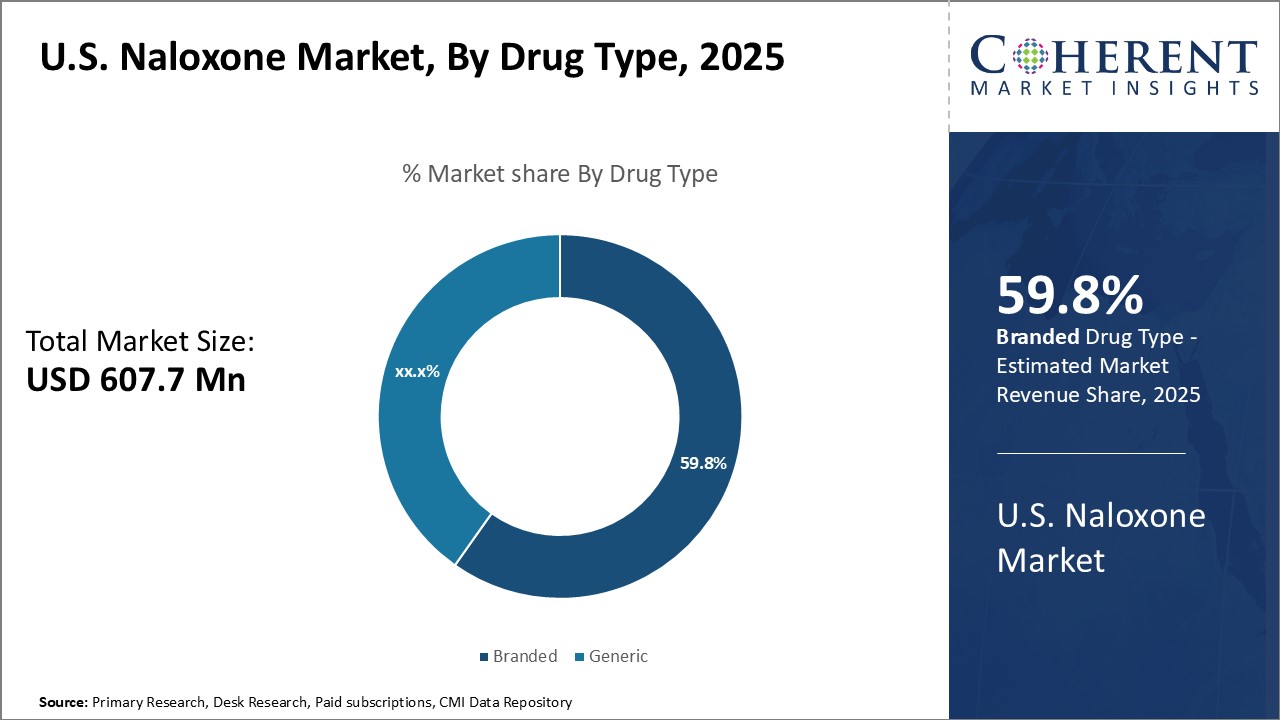
U.S. naloxone market is estimated to be valued at USD 607.7 Mn in 2025 and is expected to reach USD 1,215.5 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 10.4% from 2025 to 2032. Increasing prevalence of drug overdoses, rising healthcare expenditure, government initiatives to curtail opioid crisis, and introduction of generic drugs can drive the market growth during the forecast period.
Discover market dynamics shaping the industry: Download Free Sample
The naloxone market is expected to witness growth due to increasing incidence of opioid abuse disorders and overdose deaths in the U.S. Growing awareness regarding overdose emergencies and availability of various delivery forms such as auto-injectors and nasal sprays for emergency treatment can boost adoption of naloxone.
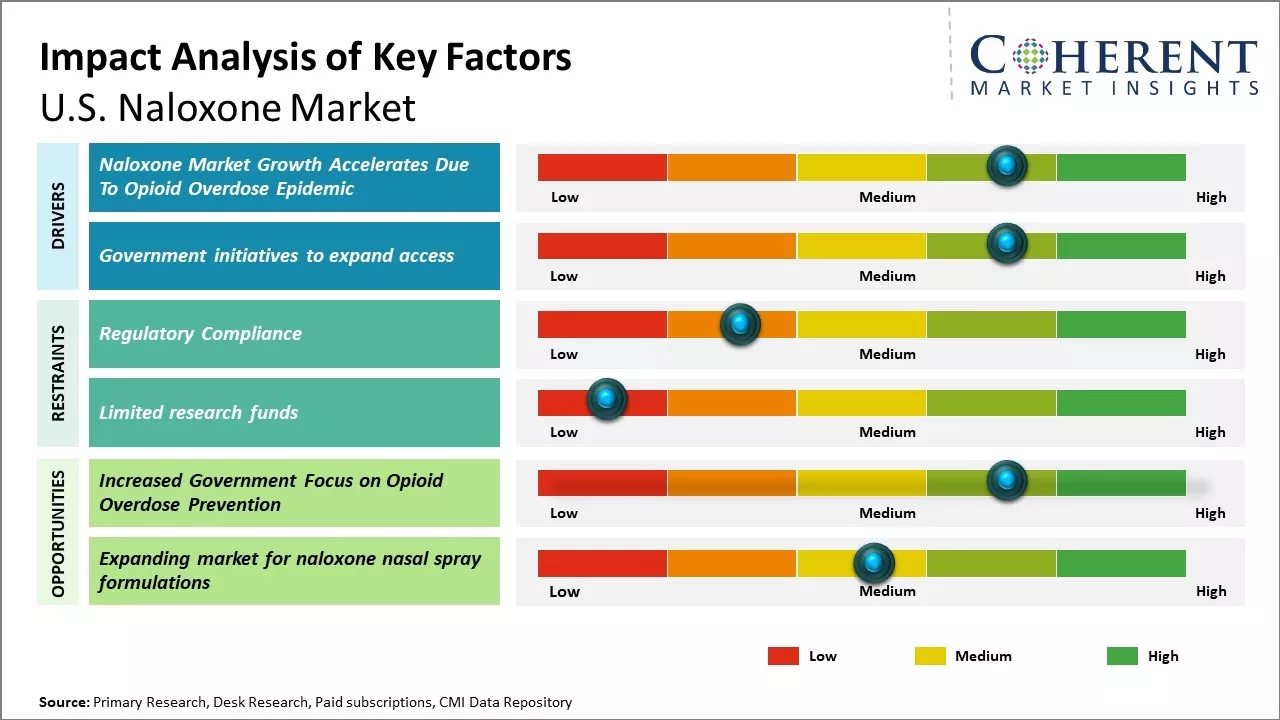
Opioid Overdose Epidemic
Growing opioid overdose epidemic in the U.S. can drive the naloxone market growth. Naloxone is a lifesaving medication that can rapidly reverse opioid overdoses. As more lives are being lost to opioid overdoses, the need for widespread access and administration of Naloxone has never been greater. According to the data published by Centers for Disease Control and Prevention, over 93,000 people died of drug overdoses in 2020, with majority of deaths being caused by synthetic opioids including fentanyl. As per the data from CDC, overdose deaths had increased almost 30% from 2019 to 2020, underlining the severity of the crisis. In response to this public health emergency, government agencies and community organizations across many U.S. states have stepped up efforts to make Naloxone more available to people at risk as well as to family members and friends of people suffering from addiction. Naloxone distribution programs have been established, and standing orders issued, allowing pharmacists to dispense Naloxone without an individual prescription. Several states have also passed legislation granting legal protection to bystanders who administer Naloxone. Such policy measures have significantly bolstered the penetration of Naloxone across communities. Moreover, educational programs about opioid overdose prevention and the use of Naloxone have raised awareness.
Get actionable strategies to beat competition: Download Free Sample
Government initiatives to expand access
The U.S government has taken several initiatives to expand access to naloxone, and this drives the naloxone market growth. Naloxone is an opioid antagonist that is used to reverse opioid overdoses, and it can save lives during an overdose emergency. The ongoing opioid crisis in the U.S. compelled government agencies to focus on increasing the availability of naloxone nationwide. For example, through programs like State Opioid Response (SOR) grants, Substance Abuse and Mental Health Services Administration (SAMHSA) has awarded billions in funds to state governments to support opioid treatment and prevention efforts. This funding will be used to exapand naloxone distribution and teach overdose prevention education. As a result, all 50 U.S. states now have standing orders or other policies that allow pharmacists to dispense naloxone without an individual prescription. Community-based programs partnering with health departments, police and EMS have also received funding to set up naloxone distribution sites across cities and towns. These efforts are steadily increasing access to and utilization of naloxone. According to the data from SAMHSA, the number of naloxone kits distributed through their funded programs had increased from under 700,000 in 2017 to over 2.6 million in 2020. As per CDC Provisional Data, EMS administrations of naloxone for suspected opioid overdoses doubled from just under 50,000 in 2016 to over 140,000 in 2021.
Key Takeaways from Analyst:
The U.S. naloxone market growth is driven by rising prevalence of opioid overdose cases in the U.S. Naloxone has emerged as the most effective life-saving treatment for reversing the effects of opioid overdose. Growing awareness among first responders and caregivers about the efficacy of naloxone in such medical emergencies can boost its demand. Furthermore, increased access to naloxone through expanded distribution programs by federal and state agencies makes drug more widely available in both healthcare and non-healthcare settings.
Lower prescription rates of naloxone products as compared to other countries with opioid crises can hamper the market growth. Relatively higher product costs as compared to other therapeutics can also hamper the market growth. Ongoing initiatives to make naloxone more affordable and accessible through public funding programs can drive the market growth.
Northeast region currently dominates market due to higher incidence rates of drug overdoses in states like New York and New Jersey. However, tWest region is anticipated to offer lucrative opportunities with states like California and Washington implementing progressive naloxone access policies.
Market Challenges; Regulatory Compliance
U.S. naloxone market growth can be hampered by complex and varying regulations around prescribing and dispensing naloxone across different states. While most states have now adopted laws expanding access to naloxone without a prescription from a medical professional, some require a prescription from a doctor or physician. These regulatory differences complicate distribution and availability of naloxone products. Manufacturers and distributors have to adhere to changing regulations in each state, which increases compliance costs and paperwork. The lack of uniform rules also leads to gaps in access, with some high-risk populations still facing barriers to obtaining naloxone in their area. Establishing a single federal policy regarding over-the-counter access to naloxone products can help streamline distribution and ensure ubiquitous availability of this life-saving drug across the U.S.
Market Opportunities: Increased Government Focus on Opioid Overdose Prevention
Rising governmental focus on preventing opioid overdose deaths can offer market growth opportunities. The federal government as well as multiple states have launched programs that promote wider distribution of naloxone. For example, the U.S. Department of Health and Human Services' five-point opioid strategy aims to improve access to treatment, reduce unintentional overdoses, strengthen data collection and support cutting edge research. Substantial funding is being allocated for public health campaigns, vaccination of high-risk groups and ambulance services being equipped with naloxone. This prioritization at the policy level boosts awareness as well as demand for naloxone products. With governmental support expected to further expand in the coming years, more innovative solutions are likely to enter this market to help address the critical public health issue of opioid addiction.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
By Drug Type- Brand Loyalty and Stronger Efficacy Boosts Sales of Branded Naloxone
In terms of drug type, branded segment is expected to contribute the highest market share of, owing to brand loyalty and perceptions of stronger efficacy. Branded drugs are often seen as the gold standard as compared to generics, thus, providing advantage to pharmaceutical companies marketing these drugs. These companies invest heavily in promotional activities and physician education to promote their branded naloxone products. Doctors also tend to be more familiar with branded drugs and confident in prescribing them. Branded drug makers invest significant resources into clinical trials to refine product formulations and dosages for optimal results. This perception of enhanced efficacy encourages doctors and patients to choose branded naloxone over generics. Pharmaceutical companies emphasize their branded products' reliability in reversing overdoses based on extensive research data. Strong branding and messaging around efficacy have helped cement customer loyalty to famous naloxone brands.
By Route of Administration- Intranasal Administration Dominates
In terms of route of administration, intranasal administration segment is expected to contribute the highest market share of, due to its ease-of-use advantages over other modes. Intranasal naloxone products are designed for simple intranasal delivery without needing medical training. This makes them uniquely suitable for emergency overdose situations outside of clinical settings. Individuals can carry intranasal naloxone and self-administer or assist others during overdoses whenever needed. Intranasal naloxone acts quickly through absorption into the bloodstream via nasal tissues. Its non-invasive delivery method reduces anxiety associated with alternative injection methods. This encourages more widespread adoption of naloxone for overdose emergencies. Intranasal naloxone's advantages in ease and speed of administration have made it the preferred option for first responders and community programs aiming to expand naloxone access.
By Distribution Channel- Hospitals Drive Distribution via Pharmacies
In terms of distribution channel, hospitals pharmacies segment is expected to contribute the highest market share of, due to their central role in facilitating healthcare access. As the frontline of medical treatment, hospitals are a key source for naloxone prescriptions to reverse overdoses. Hospital pharmacies play a critical role in fulfilling these naloxone prescriptions and educating patients on proper administration. Hospital pharmacies participate in innovative distribution programs that supply naloxone to other partners tackling the overdose crisis. These provide naloxone to first responder groups, community organizations, and retail pharmacies to expand access points. Hospitals often spearhead outreach efforts in high-risk communities. Leveraging their pharmaceutical expertise and respected brand, hospital pharmacies are driving innovative models to curb overdoses through comprehensive naloxone distribution networks.
U.S. Naloxone Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 607.7 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.4% | 2032 Value Projection: | USD 1,215.5 Mn |
| Segments covered: |
|
||
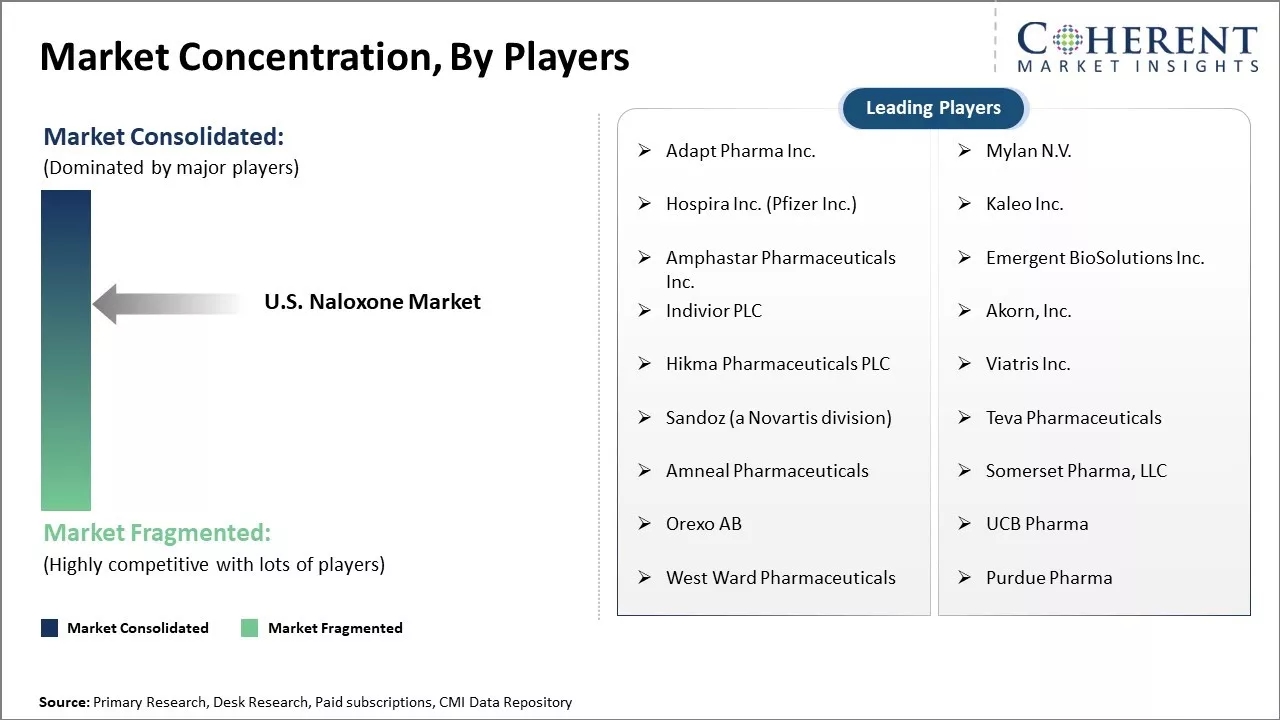
| Companies covered: |
Adapt Pharma Inc., Mylan N.V., Hospira Inc. (Pfizer Inc.), Kaleo Inc., Amphastar Pharmaceuticals Inc., Emergent BioSolutions Inc., Indivior PLC, Akorn, Inc., Hikma Pharmaceuticals PLC, Viatris Inc., Sandoz (a Novartis division), Teva Pharmaceuticals, Amneal Pharmaceuticals, Somerset Pharma, LLC, Orexo AB, UCB Pharma, West Ward Pharmaceuticals, and Purdue Pharma |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: U.S. Naloxone Market refers to sales and distribution of the drug naloxone within the U.S. Naloxone is an opioid antagonist used to reverse opioid overdoses. It competitively binds to opioid receptors and reverses and blocks the effects of other opioid drugs. As the opioid overdose crisis continues to impact communities across America, the naloxone market has grown significantly as public health officials and medical experts work to increase access to and distribution of the life-saving drug. The demand for naloxone from government agencies, hospitals, clinics, and pharmacies drives revenue in this public health-oriented market.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients