The U.S. contract research organization (CROs) market is estimated to be valued at USD 21.85 Bn in 2025 and is expected to reach USD 49.56 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 12.4% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The U.S. contract research organization (CROs) market has been witnessing positive growth over the past few years. Rising R&D expenditure of pharmaceutical and biopharmaceutical companies and the emergence of new drug pipelines with complex molecules are expected to drive the market growth during the forecast period. In addition, the growing demand for specialized testing services and strategic partnerships between pharmaceutical companies and CROs to maximize outsourcing and reduce development costs are further expected to support the market expansion. However, pricing pressures, intense competition, and lack of harmonization of regulations globally may challenge market players over the next few years. Overall, increased R&D spending and outsourcing activities indicate a promising outlook for the U.S. contract research organization (CROs) market during the forecast years.
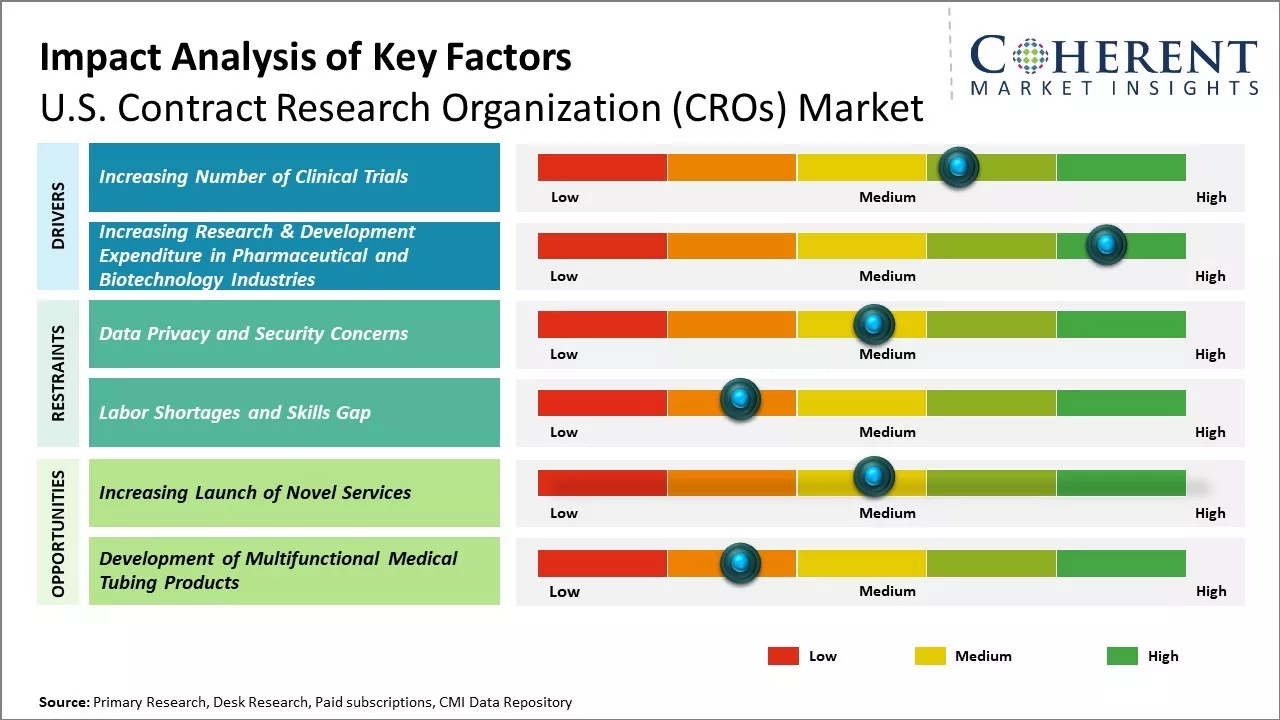
Market Driver – Increasing Number of Clinical Trials
Pharmaceutical and biotechnology companies are investing heavily in research and development (R&D) to bring new drugs and therapies to the market. As a result, there is a greater need for conducting clinical trials to test the safety and efficacy of these products. This increased research and development (R&D) expenditure boosts the demand for contract clinical research organization services. Increasing number of clinical trials globally is a key driver propelling the growth and expansion of the global contract clinical research organization market. For instance, according to the data published by Honeycomb Worldwide Inc., a company that operates in the pharmaceuticals industry, on May 17, 2023, there were 452,604 registered clinical trials globally on ClinicalTrials.gov. Out of the total registered studies, 64,838 are actively recruiting participants. This represents a significant increase from the over 365,000 registered trials reported in early 2021. It is clear that the clinical research landscape continues to expand at a substantial rate.
Get actionable strategies to beat competition: Download Free Sample
Market Driver – Increasing Research & Development Expenditure in Pharmaceutical and Biotechnology IndustriesPharmaceutical and biotechnology industries have been continuously increasing their expenditure on research and development activities, in order to introduce new drugs in the market. Companies invest huge amounts in R&D to develop advanced healthcare solutions through clinical research. However, conducting clinical trials requires specialized infrastructure and expertise that most companies may not have in-house. This boosts the global contract clinical research organization market growth. For instance, Merck & Co., Inc., a multinational pharmaceutical company, announced that its R&D expenses were US$ 9.6 billion in the fourth quarter of 2023 as compared to US$ 3.8 billion in the fourth quarter of 2022. R&D expenses were US$ 30.5 billion for 2023 as compared to US$ 13.5 billion for 2022.

To learn more about this report, Download Free Sample
Market Challenges – Data Privacy and Security ConcernsData privacy and security concerns represent a significant restraining factor for the contract clinical research organization market due to several key reasons: Complexity of Data Handling: Clinical trials generate large volumes of sensitive and personally identifiable information (PII) from participants. This includes medical history, genetic data, and other confidential details. Managing and securing such complex datasets require specialized expertise and resources. International Data Transfers: Many clinical trials are conducted across multiple countries, necessitating the transfer of data across borders. Compliance with diverse data protection laws and regulations in different jurisdictions adds complexity and potential legal risks for CROs. Ethical Considerations: Protecting the privacy and confidentiality of participants is an ethical imperative in clinical research. Any compromise in data security not only violates ethical principles but also undermines the integrity of the research process.
Market Opportunities – Increasing Adoption of Inorganic Growth Strategies by Key Players
Key market players are focused on adopting inorganic growth strategies, such as partnership agreements, in order to expand their presence globally. For instance, in June 2023, ITOCHU Corporation, a company involved in domestic trading, import/export, and overseas trading of various products, announced that its subsidiary, A2 Healthcare Corporation, had signed the first partnership agreement with NRG Oncology-Japan a non-profit organization dedicated to supporting clinical trials. NRG promotes multicenter joint clinical research within Japan as an affiliate of the National Cancer Institute. Based on the agreement, A2 Healthcare will continue to engage in the clinical trial support business for drugs that have not yet been approved in Japan to address drug lag and drug loss, which are recent social issues.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
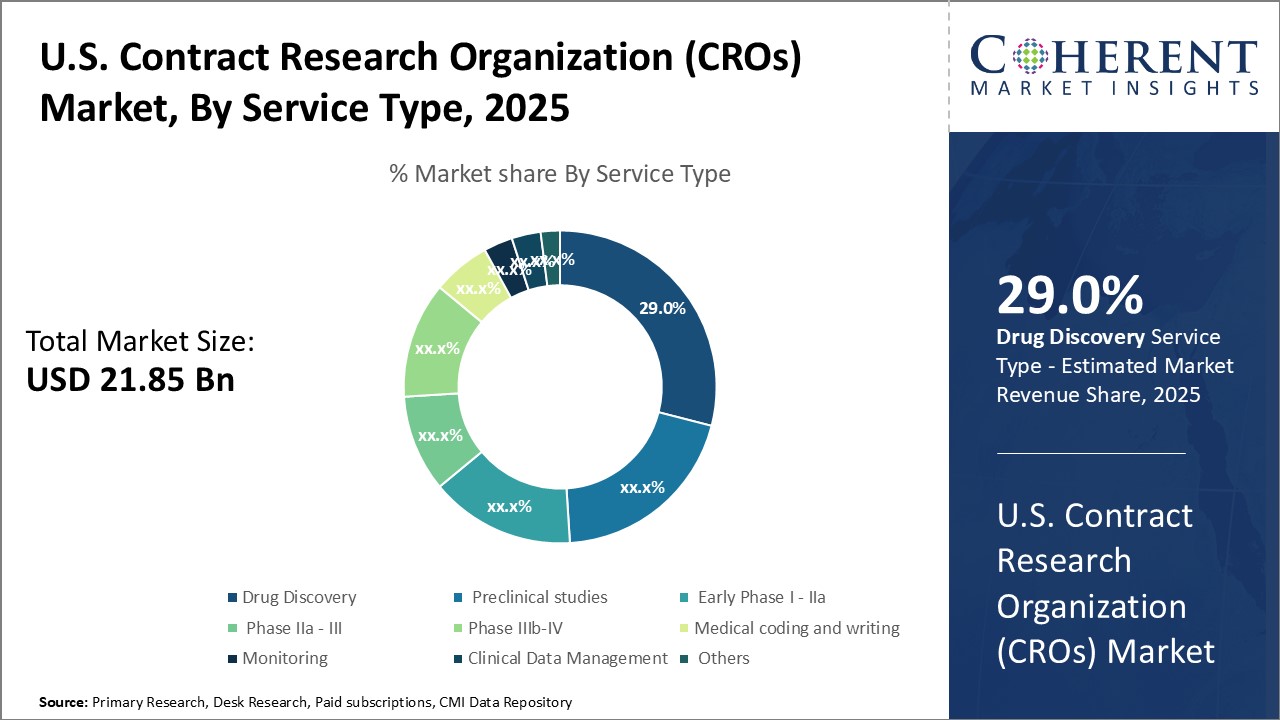
Insights, By Service Type: Rising Outsourcing and Research Investments Fuel Growth in Drug Discovery ServicesThe service type segment includes drug discovery, preclinical studies, early phase I - IIa, phase IIa - III, phase IIIb – IV, medical coding and writing, monitoring, clinical data management, and others. The drug discovery subsegment is expected to have 29.0% of the market share in 2025 owing to growing outsourcing of drug discovery activities and increasing research and development investments by pharmaceutical companies. CROs offer end-to-end drug discovery services right from target identification and validation to lead optimization. They employ advanced technologies such as genomic screening, bioinformatics and artificial intelligence which help clients accelerate the discovery process. Outsourcing drug discovery to specialized CROs allows pharmaceutical companies to focus on their core competencies while obtaining expert services in a cost-effective manner. It also provides access to latest tools and infrastructure not available in-house. Furthermore, intense patent cliff pressures on large pharma have driven them to increase discovery investments to replenish pipelines, benefiting CRO drug discovery revenues. The expertise of CROs in novel areas like oncology and rare diseases further enhances their appeal. Overall, the need to boost productivity and flexibility at reduced costs continues to support strong demand growth for outsourced drug discovery services.
Insights, By Therapeutic Application: Increasing Prevalence of Cancer
The therapeutic application segment includes oncology, cardiovascular diseases, central nervous system diseases, infectious diseases, immunological disorders, respiratory disorders, and others. The oncology subsegment is expected to have 29.8% of the market share in 2025 driven by a soaring cancer drug pipeline. With the prevalence of cancer on the rise, pharmaceutical firms are devoting huge R&D investments towards developing innovative oncology therapies. Additionally, immunotherapy and personalized medicine are opening new opportunities in this field requiring specialized expertise. CROs have emerged as strategic partners to biopharmaceutical industry in oncology with their dedicated centers housing state-of-the-art technologies and a pool of trained oncology researchers. Their multi-disciplinary approach aids faster design and execution of clinical trials. Further, their global networks help find the best suited patients and sites for studies. These advantages coupled with rising complexity of oncology studies have made CROs an invaluable resource, driving the largest revenues in the oncology segment.
Insights, Size of CROs: Extensive Service Portfolios
The size of CROs segment includes small size (less than 100 employees), medium size (100-500 employees), and large size (more than 500 employees). The large CROs (more than 500 employees) subsegment is expected to have 41.7% of the market share in 2025. Large CROs have extensive service portfolios covering the entire drug development lifecycle from pre-clinical to post-marketing. Their economies of scale allow investments in advanced lab infrastructure, large teams of specialists, and a global delivery footprint. This enables centralized project management of even the most complex multi-country studies. Large CROs also have robust quality systems and regulatory compliance processes certified by global health authorities. Furthermore, their financial might supports constant innovation through strategic acquisitions and partnerships. Pharmaceutical clients therefore prefer large trusted CROs to handle their most high-profile programs globally. In addition, large CROs have dominated the market through organic as well as inorganic growth over the past decades to cement their leadership positions.
U.S. Contract Research Organization (CROs) Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 21.85 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.4% | 2032 Value Projection: | USD 49.56 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
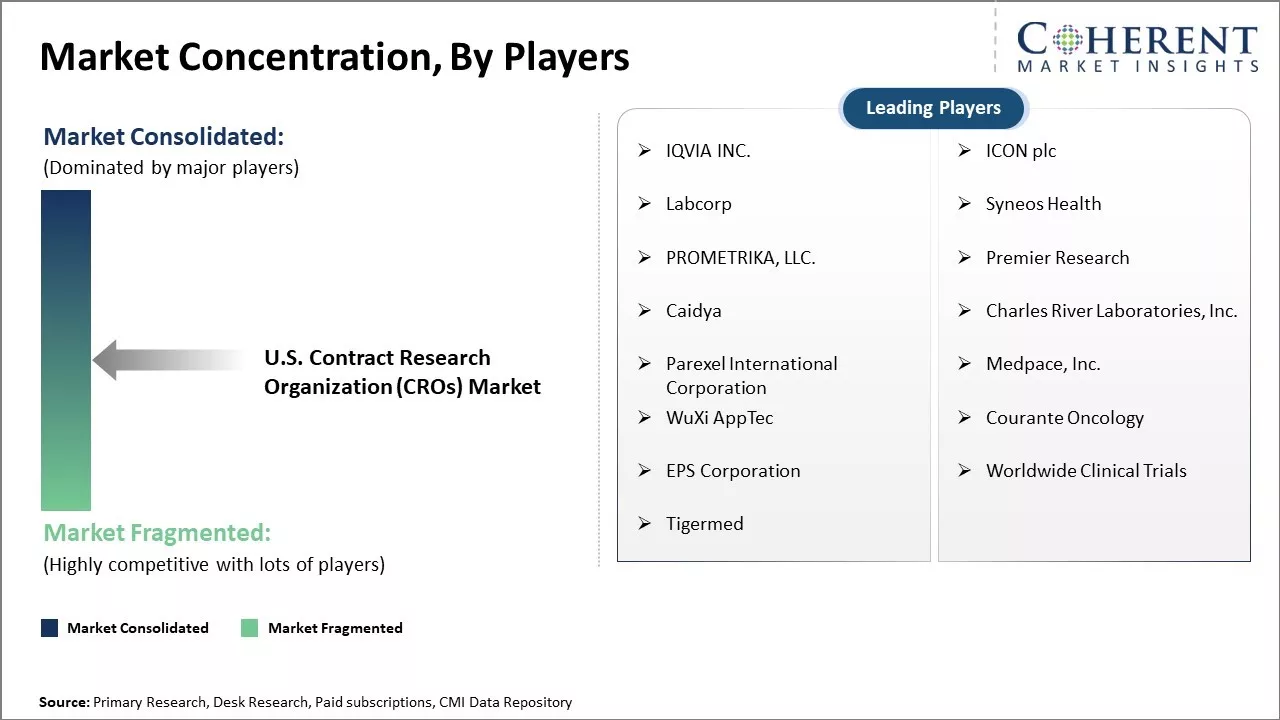
| Companies covered: |
IQVIA INC., ICON plc, Labcorp, Syneos Health, PROMETRIKA, LLC. , Premier Research, Caidya, Charles River Laboratories, Inc., Parexel International Corporation, Medpace, Inc., WuXi AppTec, Courante Oncology, EPS Corporation, Worldwide Clinical Trials, and Tigermed |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: The U.S. Contract Research Organization (CROs) Market involves companies that provide outsourced clinical trial and drug development services to pharmaceutical, biotechnology, and medical device companies. CROs take over portions of the drug development process, including study protocol design, patient recruitment, clinical evaluations, and regulatory submissions. By outsourcing various stages of clinical testing to well-established CROs, biopharma companies can control costs and focus internal resources on core drug development.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients