U.S. central venous catheter market is estimated to be valued at USD 1,142.4 Mn in 2025 and is expected to exhibit a CAGR of 6.4% during the forecast period (2025-2032). Central venous catheters play a major role in modern healthcare. These catheters allow easy administration of intravenous fluids, blood products, medications, and parenteral nutrition, as well as offer hemodialysis access and hemodynamic monitoring. They are used in various diseases for longer duration therapies such as cancer (chemotherapy) and end stage renal disease (dialysis).
U.S. Central Venous Catheter Market – Impact of Coronavirus (COVID-19) Pandemic
The COVID-19 pandemic and lockdown in various countries across the globe has impacted the financial status of businesses across all sectors. The private healthcare sector is one such sector, which has been majorly impacted by the pandemic. COVID-19 has also affected the economies in three main ways- by directly affecting the production and demand, by creating disruptions in distribution channels, and through its financial impact on companies and financial markets. The increase in central-line–associated bloodstream infections and blood culture contamination rates increase the market growth. For instance, in November 2020, National Center for Biotechnology Information published a report which reported that a there was a significant increase in central-line–associated bloodstream infections and blood culture contamination rates during the pandemic. Blood culture contamination rates were 19% higher during the COVID-19 period, whereas the CLABSI (central-line–associated bloodstream infections) rate during the pandemic increased by 25% in US.
Thus, impact of the Coronavirus (COVID-19) pandemic had driven the growth of the U.S. central venous catheter market during the pandemic.
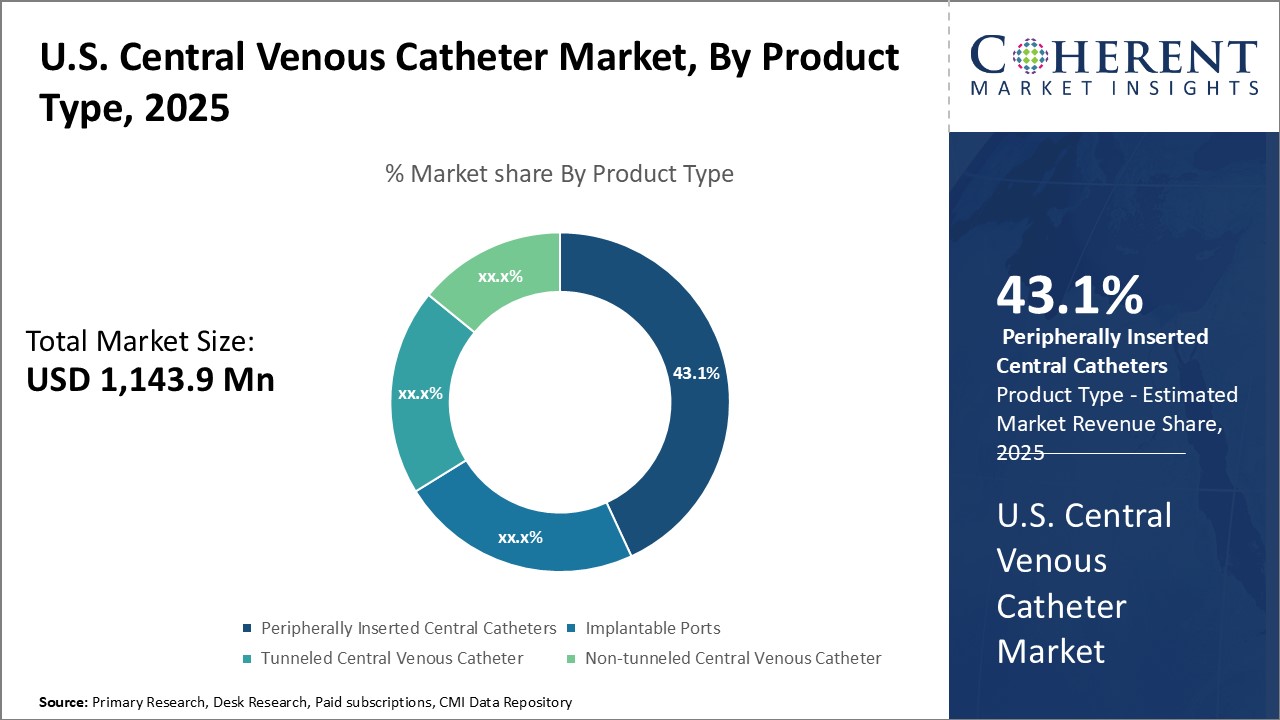
Figure 1. U.S. Central Venous Catheter Market Value (USD Mn), by Product Type, 2025
To learn more about this report, Download Free Sample
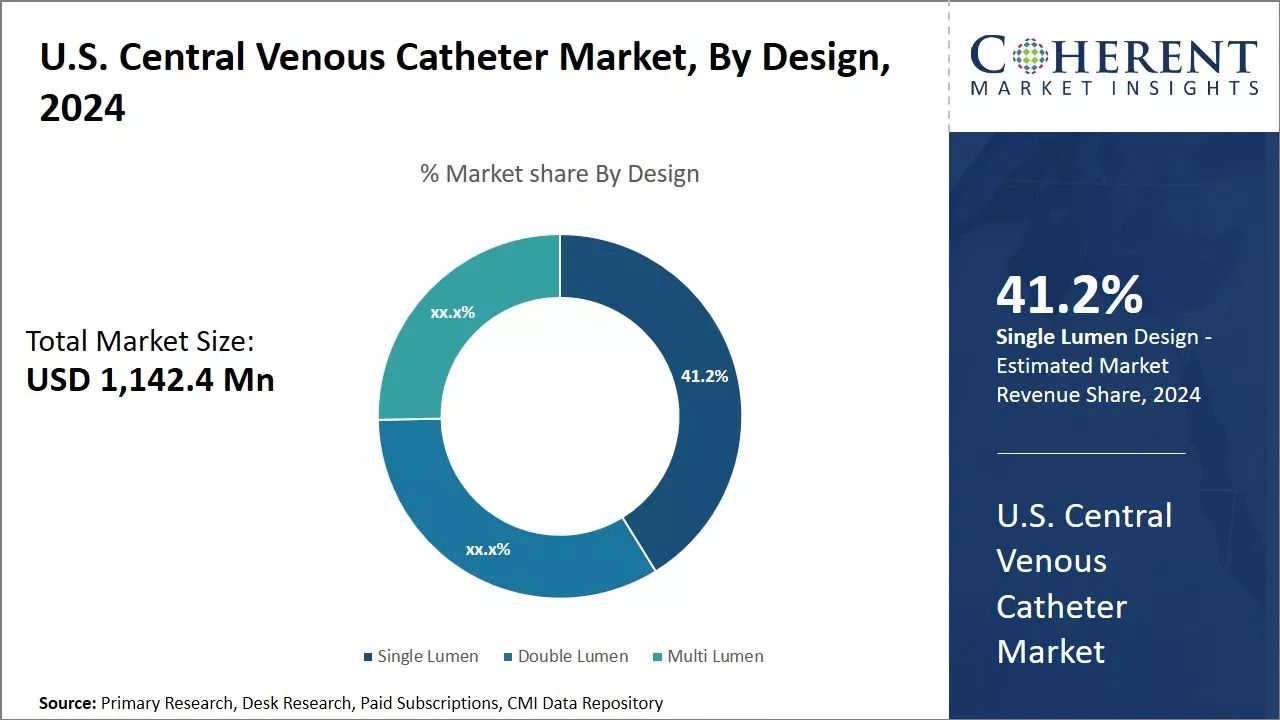
Increasing number of advantages associated with single lumen is expected to drive the growth of U.S. central venous catheter market
Increasing advantages associated with single lumen drives the growth of U.S. central venous catheter market over the forecast period. For example:
U.S. Central Venous Catheter Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1,143.9 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.4% | 2032 Value Projection: | USD 1,765.9 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
AngioDynamics, Inc, C.R. Bard, Inc, Teleflex Incorporated, B. Braun Melsungen AG, Medtronic Plc, Vygon Ltd., Cook Medical, Inc., Argon Medical Devices, Inc.,ICU Medical, Inc.,Theragenics Corporation, Becton, Dickinson and Company,icumedical, Polymedicure, Lepu Medical Technology (Beijing) Co.,Ltd., VOGT MEDICAL, Gilead Sciences, Inc and ZOLL Medical Corporation |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
U.S. Central Venous Catheter Market – Restraints
There are various restrains associated with central venous catheter market such as:
Figure 2. U.S. Central Venous Catheter Market Share, By Design, 2025
To learn more about this report, Download Free Sample
Recent Developments
In November 2023, CorMedix Inc., a biopharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) has approved DefenCath (taurolidine and heparin) catheter lock solution (CLS) to reduce the incidence of catheter-related bloodstream infections (CRBSIs) in a limited population of adult kidney failure patients receiving chronic hemodialysis via a central venous catheter (CVC).
In June 2023, Merit Medical Systems, Inc., a global medical device manufacturer, announced that it has completed the purchase of Bluegrass Vascular Technologies, Inc.'s Surfacer Inside-Out Access Catheter System for a total cash consideration of USD 32.5 million. The Surfacer System is a life-saving device for hemodialysis patients on catheters, as well as patients with other illnesses requiring venous access, who experience central venous occlusions.
In August 2022, Teleflex Incorporated, a leading global provider of medical technologies, announced that it has been granted a contract by Vizient, Inc., the largest member-driven healthcare performance improvement firm in the U.S., to deliver Central Venous Access products. Vizient Inc. offers solutions and services that improve the delivery of high-value care by harmonizing cost, quality, and market performance for more than half of the country's acute care providers.
Increasing use of peripherally inserted central catheters is expected to bolster the U.S. central venous catheter market growth over the forecast period.
Increasing use of peripherally inserted central catheters associated with low complications is expected to drive the growth of U.S. central venous catheter market over the forecast period. For instance, in May 2021, Journal of Medicine and Life, a peer-reviewed open access journal, published a report which stated that PICCs (peripherally inserted central catheters) may be used as a safe option for central venous access in children either in the intermediate- or long-term. New types of PICC may facilitate broader indications and provide longer dwell times. PICC placement can be learned easily, usually requires light sedation and/or local anesthesia, and seldom causes serious perioperative risks. Application of assisted visualization and having well-educated staff could improve the insertion success rate and reduce the complications of PICCs.
U.S. Central Venous Catheter Market - Competitive Landscape
Key players operating in the global osteoporosis drugs market include AngioDynamics, Inc, C.R. Bard, Inc, Teleflex Incorporated, B. Braun Melsungen AG, Medtronic Plc, Vygon Ltd., Cook Medical, Inc., Argon Medical Devices, Inc.,ICU Medical, Inc.,Theragenics Corporation, Becton, Dickinson and Company,icumedical, Polymedicure, Lepu Medical Technology (Beijing) Co.,Ltd., VOGT MEDICAL, Gilead Sciences, Inc and ZOLL Medical Corporation
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients