U.S. Acellular Dermal Matrices Market is estimated to be valued at USD 3.44 Bn in 2025 and is expected to reach USD 7.14 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 11% from 2025 to 2032.
Analysts’ Views on the U.S. Acellular Dermal Matrices Market:
Increasing research and development (R&D) activities by the key market players is expected to drive the growth of the U.S. acellular dermal matrices market over the forecast period. For instance, in April 2022, Memorial Sloan Kettering Cancer Center, a cancer treatment and research institution based in New York, U.S., initiated a clinical trial titled “A Randomized Controlled Trial of Prepectoral Breast Reconstruction With and Without Acellular Dermal Matrix”. The study is currently in phase 3 which is estimated to get completed in March 2025.
Figure 1. U.S. Acellular Dermal Matrices Market Share (%), By Application, 2025
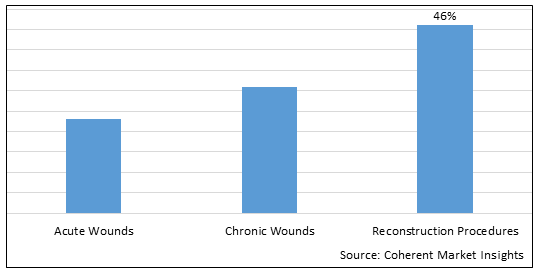
To learn more about this report, Download Free Sample
U.S. Acellular Dermal Matrices Market - Drivers
Figure 2. U.S. Acellular Dermal Matrices Market Share (%), By Origin, 2025
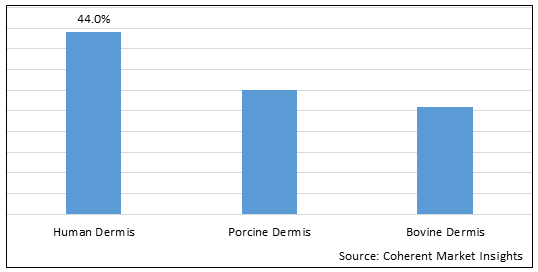
To learn more about this report, Download Free Sample
U.S. Acellular Dermal Matrices Market - Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease spread to over 100 countries across the globe, and the World Health Organization (WHO) declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. It affected specialized medical services in unprecedented ways. Surgical decision making which is always regarded as the most important aspect of care undertook an added layer of complexity in the face of the COVID-19 pandemic. This pandemic placed a strain on healthcare systems and providers, as well as forcing difficult choices about whether care can and should be delayed or reprioritized. The need to dedicate major economic, infrastructural, and medical resources to the assistance of critically ill COVID-19 patients is causing a redistribution of the activities of several medical disciplines not primarily involved in the management of COVID-19 patients.
COVID-19 negatively impacted the U.S. acellular dermal matrices market as the pandemic placed a strain on the healthcare systems and providers, as well as forced difficult choices about whether care should be delayed or reprioritized. Many surgeries were delayed or canceled due to the pandemic, which affected the U.S acellular dermal matrices market negatively.
U.S. Acellular Dermal Matrices Market Report Coverage`
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3.44 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 11% | 2032 Value Projection: | USD 7.14 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Integra LifeSciences Corporation, AbbVie Inc., Johnson & Johnson, HansBioMed, Becton, Dickinson and Company, Cook Group, Smith & Nephew Plc., Reprise Biomedical, Organogenesis Holdings Inc., Tissue Regenix, LifeNet Health, Zimmer Biomet Holdings, Inc., Stryker Corporation, MiMedx Group, PolyNovo Limited, Fidia Pharma USA Inc., Baxter International Inc., In2Bones Global, AlloSource |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
U.S. Acellular Dermal Matrices Market Segmentation:
U.S. acellular dermal matrices market is segmented into origin, application, place of setting, and region.
U.S. Acellular Dermal Matrices Market: Key Developments
U.S. Acellular Dermal Matrices Market: Trends
Increasing adoption of inorganic growth strategies such as agreement: Increasing adoption of inorganic growth strategies, such as agreements, by the key market players is expected to drive the growth of the U.S acellular dermal matrices market over the forecast period. For instance, on March 22, 2023, Aziyo Biologics, Inc, a company that develops and commercializes biologic products to improve compatibility between medical devices and the patients who need them, and Sientra, Inc., a medical aesthetics company focusing on plastic surgery, announced that they have entered into an agreement to expand the distribution of Aziyo’s SimpliDerm product line. Under the agreement terms, Aziyo Biologics, Inc will grant Sientra, Inc., certain non-exclusive rights in the U.S. to market, sell, and distribute Aziyo’s SimpliDerm for selective use in reconstruction surgery. SimpliDerm is a pre-hydrated human acellular dermal matrix (hADM) that uses a proprietary process to preserve key growth factors of native dermis that supports faster integration and more rapid revascularization while demonstrating a lower risk of inflammatory response.
U.S. Acellular Dermal Matrices Market: Restraint
U.S. Acellular Dermal Matrices Market - Key Players
The major players operating in the U.S. acellular dermal matrices market includes Integra LifeSciences Corporation, AbbVie Inc., Johnson & Johnson, HansBioMed, Becton, Dickinson and Company, Cook Group, Smith & Nephew Plc., Reprise Biomedical, Organogenesis Holdings Inc., Tissue Regenix, LifeNet Health, Zimmer Biomet Holdings, Inc., Stryker Corporation, MiMedx Group, PolyNovo Limited, Fidia Pharma USA Inc., Baxter International Inc., In2Bones Global, and AlloSource.
Definition: Acellular dermal matrix (ADM) is a soft connective tissue graft generated by a decellularization process that preserves the intact extracellular skin matrix. Upon implantation, this structure serves as a scaffold for donor-side cells to facilitate subsequent incorporation and revascularization.
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients