Drug compounding is the process of combining, mixing, or altering ingredients to create a medication tailored to the needs of an individual patient. Compounding includes the combining of two or more drugs, and compounded drugs are not FDA approved.
A drug may be compounded for a patient who cannot be treated with an FDA approved medication, such as patients who has an allergy to a certain dye and needs a medication to be made without it, or an elderly patient or a child who cannot swallow a tablet or capsule and needs a medicine in a liquid dosage form. Practitioners in hospitals, clinics, and other health care facilities sometimes provide compounded drugs to patients when an FDA approved drug is not medically appropriate to treat them.
Compounding commonly occurs in pharmacies, it may also occur in other settings, federal law addresses compounding by a licensed pharmacist in a state licensed pharmacy, or federal, or by a physician, as well as compounding by or under the direct supervision of licensed pharmacist in an outsourcing facility.
The U.S 503B compounding pharmacies market is estimated to be valued at US$ 985.6 million in 2022 and is expected to exhibit a CAGR of 7.6 % over the forecast period (2022-2030)
Figure 1. U.S 503B Compounding Pharmacies Market Value (US$ Mn), By Packaging Type, 2022
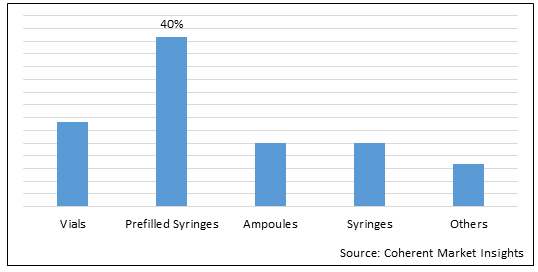
To learn more about this report, Download Free Sample
Increasing availability of compounded preparation is expected to drive the market growth over the forecast period
For instance, in May 2020, Central Admixture Pharmacy Services, Inc. launched three new compounded sterile preparations, which are in short supply for ICU patients, the products are two vasodilators: Norepinephrine 8 mg added to 250 ml normal saline (NS), Vasopressin 50 units added to 50 ml NS and sedative: Midazolam 125 mg added to NS (1mg/ml)
Moreover, in November 2019, West Pharmaceuticals Services, Inc., a 503B outsourcing facility launched Ready Pack containment system, consisting of sterile, ready-to-use (RU) NovaPure stoppers, Daikyo Crystal Zenith cyclic olefin polymer vials and Flip-Off (clean, certified, sterilized) CCS seals.
U.S. 503B Compounding Pharmacies Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 985.6 Mn |
| Historical Data for: | 2017 to 2021 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 7.6 % | 2030 Value Projection: | US$ 1,766 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Central Admixture Pharmacy Services, Inc., Nephron Pharmaceuticals Corporation, QuVa Pharma, Olympia Pharmacy, ASP Cares, Fagron Compounding Pharmacies, Athenex, Inc., Avella Specialty Pharmacy, Atlas Pharmaceuticals, Empower Pharmacy, Carie Boyd’S Prescription Shop, Edge Pharma, Imprimis NJOF, LLC, IntegraDose Compounding services, LLC, Wells Pharma of Houston, LLC, US Compounding Inc., and SCA Pharma. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2. U.S. 503B Compounding Pharmacies Market Share (%), By Molecule Type, 2022
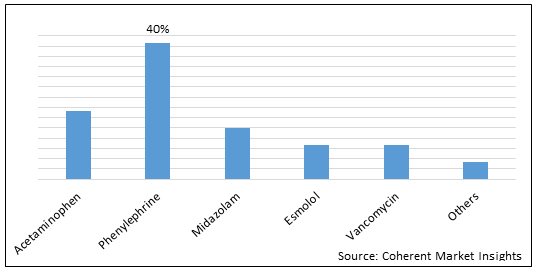
To learn more about this report, Download Free Sample
Increasing facility expansion by the market players is expected to drive the market growth over the forecast period.
For instance, in September 2019, Quva Pharma, a FDA registered manufacturer of compounded medicines announced that it completed the facility expansion of the cGMP compliant 503B, the U.S. FDA registered manufacturing facility at Bloomsbury, New Jersey, U.S, and this has increased the facility by 160,000 square feet.
Moreover, in August 2021, Empower pharmacy, a compounding pharmacy which based in Texas, U.S. announced the grand opening of its new facility in Houston, U.S. This facility will allow Empower pharmacy to prepare thousands of custom prescription each day for millions of patients across the country.
U.S 503B Compounding Pharmacies Market – Impact of Coronavirus (COVID-19) Pandemic
The coronavirus (COVID-19) outbreak was first reported on December 31, 2019, in Wuhan, China. The World Health Organization declared COVID-19 a pandemic on March 11, 2020. According to the Coronavirus (COVID-19) Weekly Epidemiological Update by the World Health Organization, over 546,357,444 cases of coronavirus disease (COVID-19) were reported till 2 July, 2022, across the globe.
In the times of covid-19 pandemic pharmacies are struggling to fulfil the demand of the medicine. Consequently, 503B outsourcing facilities are proving to be crucial partners to health systems and hospital pharmacies in helping to fulfil drug scarcity and the impact on patients during the covid-19 pandemic. The Drug Quality and Security Act, passed in 2013, allowed 503B outsourcing facilities to compound certain nonsterile and sterile drugs products in anticipation of need by providers and based on meeting certain standards required by the U.S. FDA, with massive demand surged for critical care medicines because of the pandemic, 503B facilities can supplement hospitals when commercial manufacturers cannot supply.
Market Restraint
Increasing product recalls is expected to hamper the growth of the U.S. 503B compounding pharmacies market. For instance, in August 2019, the U.S Food and Drug Administration request recall of sterile compounded drug products produced by Pacifico National Inc., an outsourcing facility doing business as AmEx pharmacy, in Melbourne, Florida. The drugs which included compounded ophthalmic products among other drug products cause unnecessary risks due to significant quality and sterility concerns.
Key Players
Major players operating in the U.S. 503B compounding pharmacies market include Central Admixture Pharmacy Services, Inc., Nephron Pharmaceuticals Corporation, QuVa Pharma, Olympia Pharmacy, ASP Cares, Fagron Compounding Pharmacies, Athenex, Inc., Avella Specialty Pharmacy, Atlas Pharmaceuticals, Empower Pharmacy, Carie Boyd’S Prescription Shop, Edge Pharma, Imprimis NJOF, LLC, IntegraDose Compounding services, LLC, Wells Pharma of Houston, LLC, US Compounding Inc., and SCA Pharma.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients