Urinary Incontinence Treatment Devices Market Size and Forecast – 2025 – 2032
The Global Urinary Incontinence Treatment Devices Market size is estimated to be valued at USD 2.1 billion in 2025 and is expected to reach USD 3.8 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 8.2% from 2025 to 2032.
Global Urinary Incontinence Treatment Devices Market Overview
Urinary incontinence treatment devices encompass a wide range of medical technologies designed to manage or treat involuntary urinary leakage. These include urethral slings, artificial urinary sphincters, catheters, electrical stimulation devices, and pessaries, each targeting specific forms of stress, urge, or mixed incontinence. Minimally invasive sling systems provide support to the urethra to restore continence, while advanced neuromodulation devices regulate bladder nerve pathways to improve control. Catheters and absorbent care products offer non-surgical, immediate management solutions, whereas implantable artificial sphincters provide long-term functional restoration. These devices are engineered for safety, biocompatibility, and durability, with technological advancements increasingly focused on patient comfort and long-term efficacy.
Key Takeaways
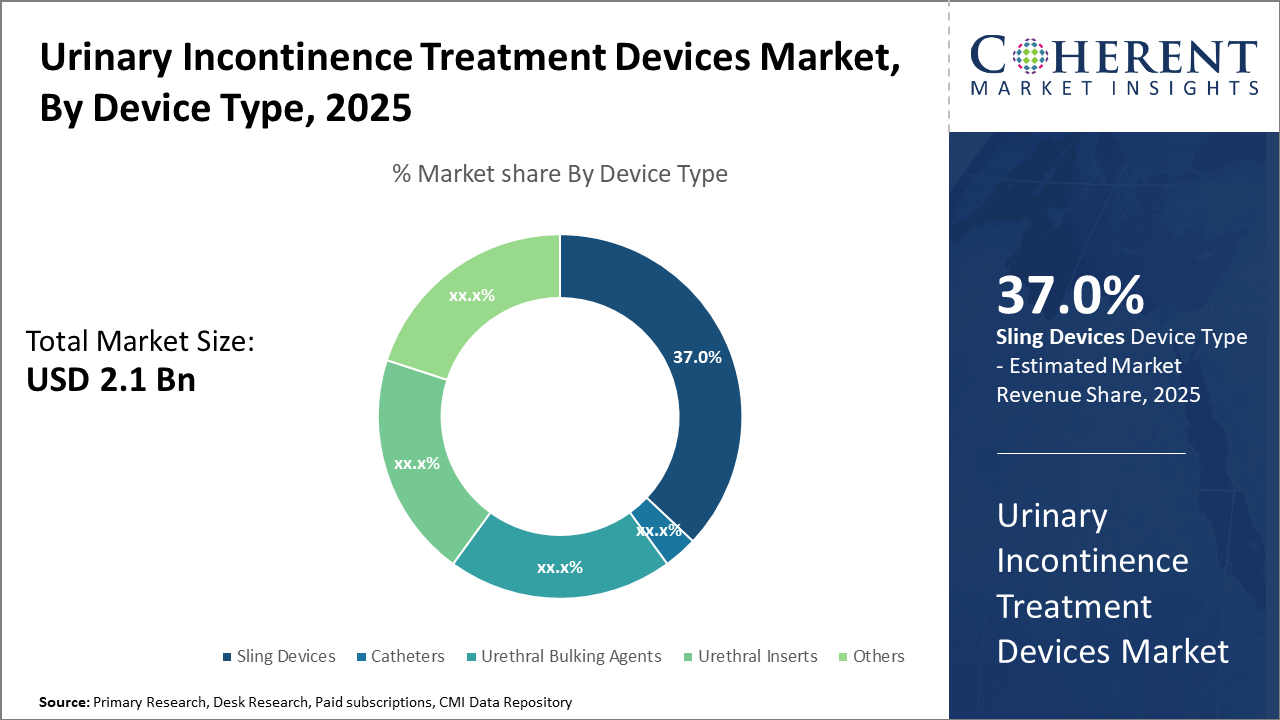
The Sling Devices segment dominates with a market share of 37%, driven by their widespread clinical acceptance and high efficacy, while Non-Invasive Devices are the fastest-growing due to improved patient compliance and reduced complications.
Specialized Urology Clinics hold the largest revenue share in the end-user category, underscoring the importance of expert care facilities in delivering treatment.
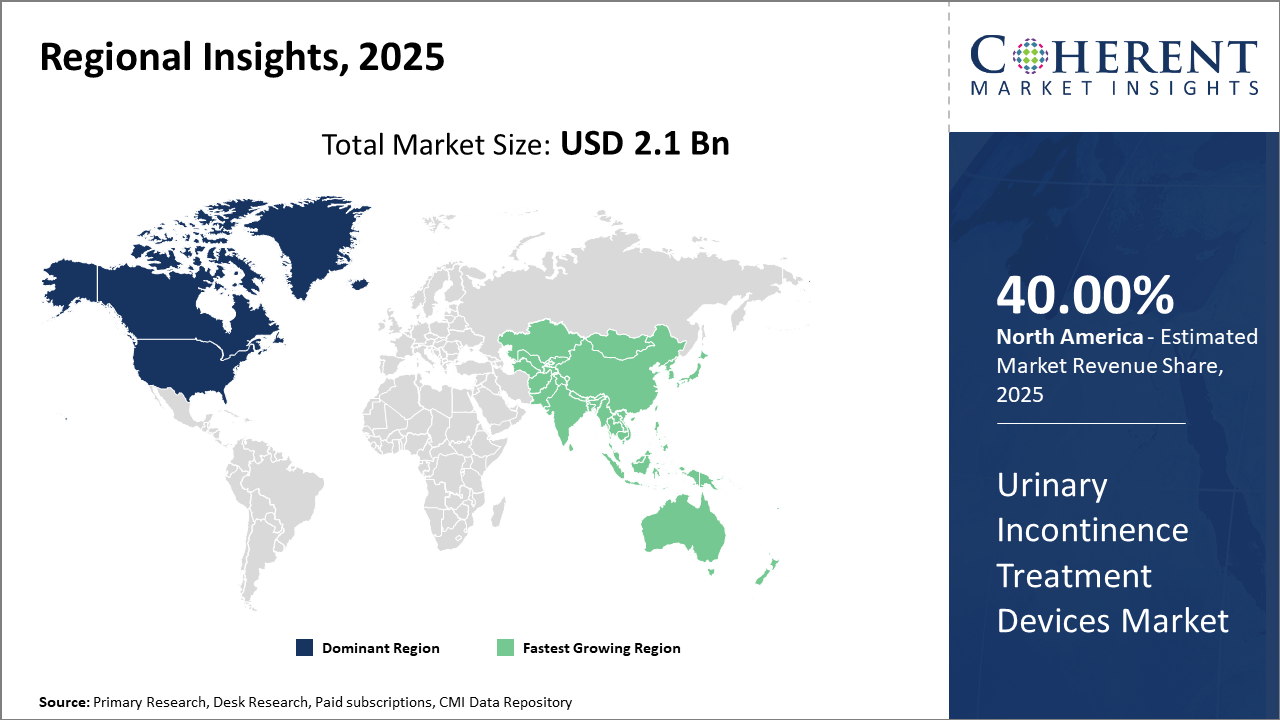
Regionally, North America continues to command industry share owing to advanced healthcare infrastructure and favorable reimbursement policies.
Asia Pacific exhibits the fastest CAGR, fueled by growing healthcare investments and increasing awareness campaigns.
Europe maintains steady growth attributed to consistent demand and regulatory support for medical device innovations.
Urinary Incontinence Treatment Devices Market Segmentation Analysis
To learn more about this report, Download Free Sample
Urinary Incontinence Treatment Devices Market Insights, By Device Type
Sling Devices dominate the market share due to their proven efficacy in treating stress urinary incontinence, with innovations like adjustable slings improving patient satisfaction significantly. The fastest-growing subsegment is Catheters, driven by developments in intermittent and indwelling catheter technologies that reduce infection risks and enhance user comfort. Urethral Bulking Agents provide minimally invasive options primarily for patients unsuitable for surgery, while Urethral Inserts offer temporary management solutions.
Urinary Incontinence Treatment Devices Market Insights, By Technology
Implantable Devices are dominating the market share. Implantable devices are preferred for their effectiveness and longer-term relief, supported by recent innovations such as bioelectronic implants introduced in 2025 that enhance bladder control accuracy. Non-Invasive Devices are the fastest-growing segment, leveraging patient preference for less intrusive treatments and rise in outpatient care settings. Injectable Agents provide localized treatments with fewer side effects. Behavioral and Electrical Stimulation Devices, including neuromodulation, aid in managing complex cases.
Urinary Incontinence Treatment Devices Market Insights, By End-User
Specialized Urology Clinics dominate the market share. These clinics offer specialized expertise, enabling higher successful treatment rates and adoption of advanced devices. The fastest growing subsegment is Home Care Settings, powered by telehealth expansion and increased patient preference for at-home management, especially post-2024. Hospitals provide critical inpatient services and often lead in conducting clinical trials for novel devices. Ambulatory Surgical Centers cater to minimally invasive procedures with faster turnaround times.
Urinary Incontinence Treatment Devices Market Trends
Current market trends focus heavily on technology-driven solutions, such as the integration of biosensors and real-time monitoring capabilities within implantable devices, providing improved patient outcomes and adherence.
The rise in home-based care facilitated by remote device monitoring via mobile applications is reshaping treatment paradigms, especially post the global push for telehealth in 2024.
Eco-friendly device materials are another evolving trend aligning with sustainability goals, gaining traction in developed regions.
Urinary Incontinence Treatment Devices Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Urinary Incontinence Treatment Devices Market Analysis and Trends
In North America, the urinary incontinence treatment devices market dominance is anchored by extensive healthcare infrastructure, strong reimbursement frameworks, and high consumer awareness, collectively accounting for over 40% market share. Major market players maintain headquarters and significant R&D centers here, fostering continuous innovation.
Asia Pacific Urinary Incontinence Treatment Devices Market Analysis and Trends
Meanwhile, Asia Pacific exhibits the fastest growth with an expected CAGR exceeding 9% fueled by increasing healthcare expenditure, rising prevalence of urological disorders, and expanding healthcare access in countries like China and India. Government initiatives to improve elderly care and growing adoption of advanced medical devices contribute heavily to this regional surge.
Urinary Incontinence Treatment Devices Market Outlook for Key Countries
USA Urinary Incontinence Treatment Devices Market Analysis and Trends
The U.S. market represents a substantial portion of the North American industry share, attributed to early adoption of innovative devices and strong insurance coverage for urinary incontinence treatments. The National Institutes of Health (NIH) statistics reported a 12% increase in device-assisted procedures in 2024 compared to 2023. Major players such as Boston Scientific and Medtronic invest heavily in R&D and clinical trials within the U.S., enhancing device efficacy and broadening treatment applicability. This impacts market growth strategies favorably while encouraging the proliferation of minimally invasive treatment options.
Japan Urinary Incontinence Treatment Devices Market Analysis and Trends
Japan’s market showcases robust growth driven by a rapidly aging population and advanced healthcare technologies. Government healthcare policies promoting elderly care and urological health screening have increased device utilization rates by nearly 10% year-over-year. Companies like Coloplast and Hollister Incorporated have augmented their presence through localized production and tailored device offerings catering to Japan’s demographic requirements. This strategic approach supports sustained market revenue growth and positions Japan as a key regional market within the Asia Pacific.
Analyst Opinion
Increasing adoption of advanced implantable devices significantly propels market revenue. Recent data from 2024 indicates that implantable devices accounted for approximately 42% of the total market share, reflecting strong supply-side dynamics influenced by increased production capabilities in Asia Pacific regions. Such devices offer minimally invasive solutions with higher efficacy, driving market growth strategies through technological innovation and enhanced patient compliance.
Demand-side indicators reveal the rise in outpatient procedures for urinary incontinence treatment as a key growth driver. In 2025, outpatient clinics reported a 15% increase in utilization of non-invasive devices compared to previous years, indicating shifting use cases and consumer preference toward convenience and reduced recovery times. This change underscores evolving market segments focused on patient-centered care models globally.
Pricing dynamics remain a critical micro-indicator; despite rising raw material costs, competitive pricing strategies among market companies have maintained accessibility for end users, especially in North America. In 2024, the average selling price of slings and catheters decreased by 3.5%, enabling enhanced penetration and amplification of industry share in cost-sensitive segments.
Export volumes from leading manufacturing hubs surged by 12% in 2024, driven predominantly by increased demand in Europe and Latin America. Rising trade activities underline market revenue growth and favorable trade dynamics, influencing long-term market forecasts and reflecting strong regional collaboration and regulatory harmonization efforts.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: |
USD 2.1 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.2% | 2032 Value Projection: |
USD 3.8 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Natus Medical Incorporated, Stryker Corporation, Coloplast Corp., Wellspect HealthCare, Teleflex Incorporated, Cook Medical, OptiMedica Corporation, TENA (Essity Group), UroGen Pharma Ltd., American Medical Systems. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Urinary Incontinence Treatment Devices Market Growth Factors
The growing geriatric population and rising prevalence of urinary incontinence worldwide stand as the primary market drivers, accounting for a substantial segment of medical device consumption. Increasing incidence rates, especially reported by healthcare bodies in North America and Europe, have resulted in heightened demand for treatment devices. Technological advancements in implantable devices and minimally invasive procedures reduce complications and enhance user acceptability, encouraging wider adoption. Additionally, expanding healthcare infrastructure and favorable reimbursement policies in regions like North America and Asia Pacific amplify market growth strategies. Rising awareness campaigns and advancements in home care settings also promote early diagnosis and treatment, contributing notably to market drivers and overall market revenue.
Urinary Incontinence Treatment Devices Market Development
In January 2024, Medtronic launched the InterStim Micro neuromodulation system, a next-generation rechargeable sacral neuromodulation device for patients with overactive bladder (OAB), urinary retention, and fecal incontinence. The system offers a significantly smaller implant size, extended battery longevity, and personalized programming options, improving patient comfort and long-term therapy adherence. It represents one of the most advanced minimally invasive neuromodulation solutions in the continence-care landscape.
In September 2025, Medtronic introduced the Altaviva™ system, an upgraded neuromodulation platform for overactive bladder designed to deliver more precise stimulation with enhanced patient-specific adjustment algorithms. The device leverages improved sensing capabilities and optimized energy delivery, allowing more accurate targeting of sacral nerves. Its launch further strengthens Medtronic’s leadership in OAB neuromodulation technologies.
In July 2024, NeoTract’s UroLift® System received U.S. FDA approval for the treatment of male stress urinary incontinence (SUI), expanding its clinical use beyond benign prostatic hyperplasia (BPH). This minimally invasive implant-based procedure provides mechanical support to improve urethral closure during physical activity. The approval positions UroLift® as a pioneering non-surgical option for men with SUI who seek rapid recovery and preservation of sexual function.
Key Players
Leading Companies of the Market
Natus Medical Incorporated
Stryker Corporation
Coloplast Corp.
Wellspect HealthCare
Teleflex Incorporated
Cook Medical
OptiMedica Corporation
TENA (Essity Group)
UroGen Pharma Ltd.
American Medical Systems
Market companies have adopted aggressive merger and acquisition strategies; for instance, Boston Scientific’s acquisition of a key urology device manufacturer in 2024 expanded its product portfolio, contributing to a 7% hike in their market share. Similarly, Kimberly-Clark’s focus on innovating catheter solutions and expanding manufacturing in Asia Pacific has bolstered competitive positioning and sped up market penetration in emerging economies.
Urinary Incontinence Treatment Devices Market Future Outlook
The market is expected to grow rapidly as non-invasive and minimally invasive devices gain preference over conventional surgeries. Technological advancements—such as next-generation electrical stimulation wearables, customizable sling systems, and smaller neuromodulation implants—will enhance patient comfort and long-term compliance. Increasing geriatric populations, especially in Asia-Pacific, will expand the patient pool. Digital health integration, continuous pelvic monitoring, and AI-based bladder assessment tools are expected to support personalized treatment pathways. Strong investments in women's health, coupled with growing acceptance of early intervention, will sustain high adoption of modern devices.
Urinary Incontinence Treatment Devices Market Historical Analysis
Historically, the urinary incontinence treatment devices market has grown steadily due to rising awareness of pelvic floor disorders and the increasing aging population in developed regions. Early growth was driven by catheters, pessaries, and absorbent products, with minimally invasive slings emerging in the 2000s as a major technological advancement. Over time, improved materials, better biocompatibility, and evidence-based surgical techniques strengthened adoption. Neuromodulation systems and artificial sphincters provided long-term solutions for complex cases, while post-pregnancy incontinence awareness campaigns and expanding women’s health programs increased early diagnosis and treatment rates. Reimbursement improvements in North America and Europe significantly shaped device penetration historically.
Sources
Primary Research Interviews:
Urologists
Gynecologists
Continence Nurses
Urodynamic Specialists
Medical Device Distributors
Databases:
WHO Health Data
GlobalData Medical Devices
CDC Health Statistics
IQVIA Procedure Volumes
National Inpatient Sample (NIS)
Magazines:
Medical Device Network
Urology Times
MedTech Innovation News
DeviceTalks Magazine
Hospital Healthcare Europe
Journals:
Journal of Urology
Neurourology and Urodynamics
Urology Journal
International Urogynecology Journal
BJU International
Newspapers:
The Washington Post (Health)
The Guardian (Health)
The Economic Times (Healthcare)
MedTech Dive News
Reuters Health
Associations:
American Urological Association (AUA)
International Continence Society (ICS)
European Association of Urology (EAU)
Medical Device Manufacturers Association (MDMA)
Federation of Gynecology and Obstetrics
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients