The global urinary catheters market is estimated to be valued at USD 6.19 Bn in 2025 and is expected to reach USD 9.37 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 6.1% from 2025 to 2032.
To learn more about this report, Download Free Sample
The urinary catheters market is experiencing significant growth driven by rising prevalence of urinary disorders, increasing geriatric population, and advancements in catheter technology. For instance, the aging global population leads to higher incidences of urinary incontinence and retention, boosting catheter demand. Additionally, growing awareness and adoption of minimally invasive procedures have propelled the use of specialized catheters like Foley and intermittent catheters. Technological innovations, such as antimicrobial coatings and biocompatible materials, enhance patient safety and comfort, further expanding market adoption. For example, the introduction of antimicrobial-coated catheters has reduced infection rates in hospitals, encouraging healthcare providers to prefer these advanced options. These factors collectively contribute to the steady expansion of the urinary catheters market worldwide.
|
Event |
Description and Impact |
|
Aging Global Population Demographics and Rising Chronic Diseases |
|
|
Regulatory Harmonization and Medical Device Safety Standards |
|
|
Technological Innovation in Smart Medical Devices and Materials |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Innovation in urinary catheters is primarily focused on enhancing safety, comfort, and monitoring capabilities to improve outcomes in urinary catheterization procedures. Unlike pharmaceuticals, urinary catheters progress through R&D, regulatory clearances such as FDA 510(k), and CE marking rather than traditional clinical trial phases.
Current pipeline developments highlight antimicrobial and infection prevention technologies, such as silver-alloy coated catheters by Teleflex and ConvaTec, and nitric oxide-releasing catheters under clinical development by Novan Inc. These innovations aim to reduce catheter-associated urinary tract infections (CAUTIs), a major challenge in urinary catheter usage in hospitals.
Material innovation also plays a key role, with hydrogel-coated catheters improving patient comfort and biocompatibility, alongside emerging biodegradable catheter components under early research. Another important area is smart/connected catheters, including IoT-enabled monitoring devices and pressure-sensing Foley catheters, which provide real-time data to enhance patient management.
In terms of materials, the ongoing debate between latex vs silicone urinary catheters persists, with silicone favored for reduced allergic reactions and increased patient tolerance. Intermittent urinary catheters continue to gain prominence due to their benefits in minimizing infection risks and supporting self-catheterization.
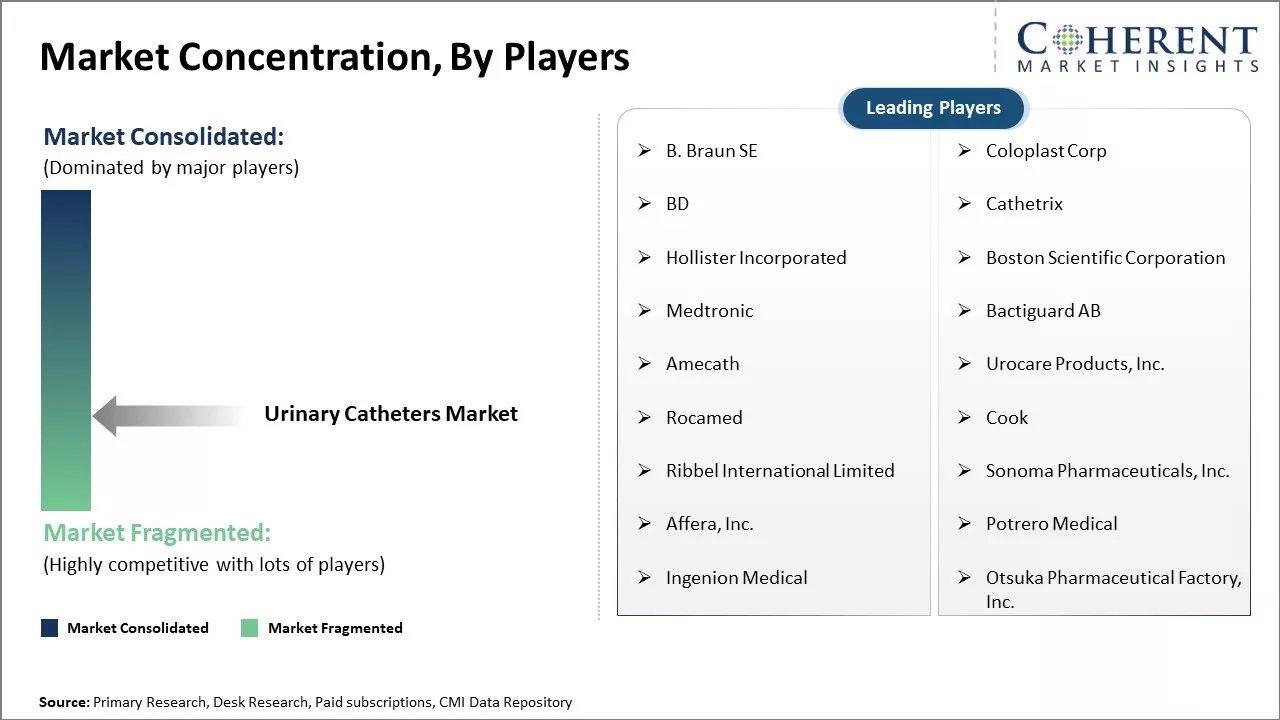
Leading companies driving these innovations include Teleflex, Coloplast, ConvaTec, BD, and Boston Scientific.
The urinary catheters market features a tight competition as a result of increased patents regarding infection control, catheter design improvement, and ensuring patients’ comfort. Many patents focus on antimicrobial silver-alloy and nitric oxide-releasing methods, made to combat catheter-associated urinary tract infections (CAUTIs). Major medical device corporations, Teleflex, Coloplast, ConvaTec, and BD, own a large portfolio of these patents creating a competitive edge for them in the market.
Also, material inventions feature patents on hydrophilic and biodegradable catheter parts to augment biocompatibility and lessen detrimental impact on the environment. Patented smart catheters with IoT and sensor-based systems for monitoring VA catheter systems show the rising attraction to digital health integration in the urinary catheters sector.
Patent applications are still mostly made in North America, Europe, and Asia-Pacific seeing as these areas have advanced research and development infrastructures and regulatory climates. There is a gradual rise in patent applications in emerging markets as manufacturers look to protect local tailored innovations. All in all, the urinary catheter patent market is still highly competitive but strategically focuses on stand out technology while aiming for evolving clinical and patient needs.
The worldwide market for urinary catheters is submerged in a reimbursement quagmire shaped by diverse coding systems, governing bodies, and insurance frameworks. In the United States, urinary catheters are reimbursed primarily under Medicare Part B as a Durable Medical Equipment. Urinary catheters are reimbursed as DME with rates between $1.20 to $25.00 per unit for intermittent, indwelling and external catheters.
Coverage necessitates medical necessity documentation, supplier enrollment, prior authorization for quantity limits which requires a declaration of need. Important reimbursement codes are HCPCS Level II A4338-A4358 for DME urinary catheters, and CPT codes 51701-51710 for the catheter insertion and management.
Medicaid coverage is state dependent, with about 38 states providing full reimbursement for catheters while some have limits with prior authorization. In Europe, reimbursement decisions are made by public health insurance systems and are influenced by the national health technology assessment bodies like NICE in the UK, G-BA in Germany, and HAS in France which directly affect catheter reimbursement.
Countries in the Asia Pacific such as Japan, China, and Australia have their reimbursement policies guided by their respective regulatory bodies PMDA, NMPA, and TGA. In summary, urinary catheter reimbursement is still spiraled in complexity and geographical disparities which restrict market access and adoption. This forces manufacturers to encounter countless regulations across different regions in order to have enduring coverage.
Urinary catheter prescriber preferences are influenced by clinical effectiveness, patient safety, hospital policies, and patient-specific requires. Distinct group practitioners such as urologists, emergency medicine, rehab physicians, and family physicians follow specific patterns aligned to the stage of care and specific pathology.
Indwelling catheters such as Bard Lubricath® and Teleflex Rusch® Gold are used in acute care settings for postoperative drainage, critical care inotrope monitoring, and management of severe incontinence. Silicone Foley catheters are often used for short-term catheterization because of their biocompatibility.
Intermittent catheters are the most prescribed devices for patients with neurogenic bladder or chronic urinary retention. Urologists and rehabilitation physicians tend to favor products such as Coloplast SpeediCath® and Hollister VaPro® because of hydrophilic coatings which lowers urethral trauma, enhancing patient compliance.
For male incontinence, dementia, or patients with limited mobility, external catheters like Hollister InView® and Coloplast Conveen® are used in non-invasive, domiciliary settings. For recurrent infections, prescribers tend to use silver-alloy, or Bardex® I.C. and Arrow® Silver Soaker™ catheters, which are proven to reduce CAUTIs by 40 to 60%, and are often prescribed with antimicrobial coatings.
To learn more about this report, Download Free Sample
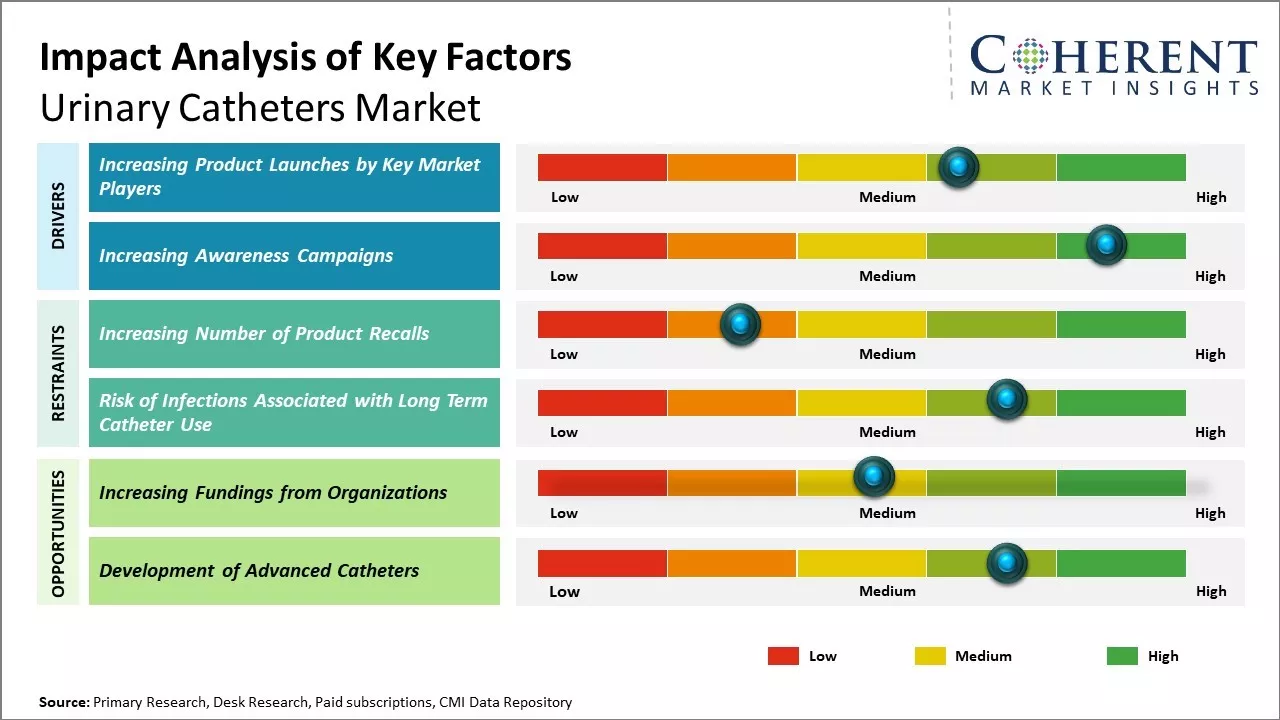
Increasing adoption of organic growth strategies, such as product launches, by key market players is expected to drive the global urinary catheters market growth over the forecast period. For instance, in December 2021, Otsuka Pharmaceutical Factory, Inc., a healthcare company, announced that it had launched Actreen, an intermittent urological catheter, which is used during self-catheterization of patients who have lost the urge to urinate or usually have difficulty urinating.
Increasing initiatives taken by the market players in collaboration with government agencies by conducting awareness campaigns for the maintenance of women hygiene may act as a driver for the market growth over the forecast period.
For instance, in May 2022, Santex Products (Pvt.) Ltd., a female hygiene product offering company, and the United Nations Children's Fund (UNICEF), in collaboration with the School Education & Literacy Department, Government of Sindh, Pakistan, announced the launch of NoChuttiPlus campaign to educate adolescent girls about Menstrual Health and Hygiene (MHH), as risks associated with poor hygiene can directly affect reproductive health and cause urinary tract infections.
Increasing number of product recalls by regulatory authorities, such as the U.S. Food and Drug Administration, is expected to hinder the market growth. For instance, in April 2020, the U.S. Food and Drug Administration recalled a class II device silicone foley catheter with a temperature sensor, which was manufactured by Covidien, a subsidiary of Medtronic, a medical device company. The device was recalled because the catheter failed to meet the established calibrated temperature sensing tolerance and correctly measure patient body temperatures that lead to an incorrect diagnosis and insufficient treatment.
Increasing adoption of inorganic growth strategies such as fundings is expected to offer lucrative growth opportunities in the market for the development of advanced urinary catheters. For instance, in August 2023, the Department of Biological Sciences at the University of Notre Dame, a private Catholic research university, Indiana, U.S. announced that it had been awarded funding from Open Philanthropy, a non-profit organization, for further development of a new type of urinary catheter that may reduce deaths from catheter-induced urinary tract infections (CAUTI).
The product type segment includes intermittent catheter, indwelling (foley) catheter, and external catheter. The intermittent catheter sub-segment is estimated to hold 54.8% of the market share in 2025 owing to its convenience and cost-effectiveness compared to other product types.
Intermittent catheters allow flexible urination and do not require permanent insertion like an indwelling catheter. This makes intermittent catheters preferable for patients needing short-term catheterization. Their use is determined by the patient's urinary habits rather than being tied to a drainage bag continuously. This flexibility allows patients to lead relatively normal lifestyles.
From a cost perspective as well, intermittent catheters have a lower unit cost compared to indwelling catheters which require longer-term use and constant maintenance. Their single-use nature also eliminates risks of complications from long-term indwelling. The convenience and affordability factors, along with their non-invasiveness, make intermittent catheters highly attractive for patients requiring temporary relief from urinary problems.
The application segment includes urinary incontinence, urinary retention, prostate gland surgeries, spinal cord injuries, and others. The urinary incontinence sub-segment is estimated to hold 39.6% of the market share in 2025 due to the growing burden of urinary incontinence. For instance, according to data published by American Urogynecologic Society, it was stated that the overall prevalence of urinary incontinence in adult U.S. women 20 years and older was 61.8%.
Urinary incontinence significantly impacts daily activities and social engagement of patients. If left untreated, it can lead to social isolation, depression and mobility issues. Catheters help regain independence and restore confidence for incontinent patients. Among all applications, urinary incontinence has wide scope for catheter usage given its association with aging, childbirth, obesity, and neurological disorders. Managing incontinence effectively is critical to uphold patients' dignity and improve their participation in social roles.
The end user segment includes hospitals, specialty clinics, ambulatory surgical centers, and others. The hospitals sub-segment is estimated to hold 43.2% of the market share in 2025 owing to their prominence in clinical excellence and enhanced access for patients. Being the preferred healthcare facilities for critical illnesses and injuries, hospitals witness high throughput of urological cases needing catheterization.
They are also increasingly focused on best practices to prevent healthcare-associated infections from improper catheter use. Furthermore, tertiary and quaternary hospital networks provide widespread coverage and role model clinical standards for other healthcare providers. This improves catheterization access for large patient volumes including those in remote areas.
To learn more about this report, Download Free Sample
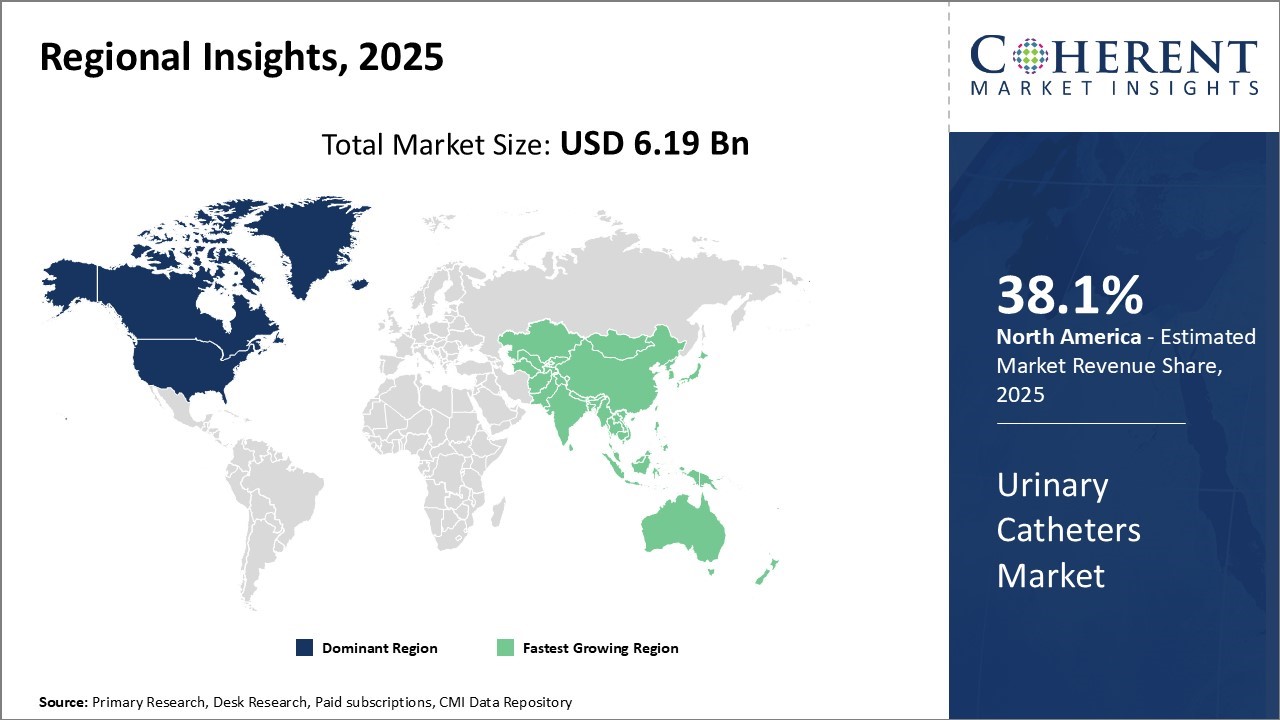
North America remains the dominant region in the global urinary catheters market, expected to hold around 38.1% of the market share by 2025. This leadership is fueled by the presence of major industry players and the region’s growing aging population, which drives demand for catheterization solutions.
The United States commands the largest share within North America, supported by a strong healthcare infrastructure, advanced catheter technologies, and favorable reimbursement policies that promote higher adoption rates. These factors collectively sustain steady market growth across the region.
The Asia Pacific (APAC) region is poised to be the fastest-growing market for urinary catheters over the forecast period. Rapid economic development, increasing healthcare investments, and expanding medical infrastructure in countries such as China, India, Japan, and South Korea are key growth drivers.
The rise in medical tourism and preference for cost-effective catheter devices further stimulate demand in the region. Additionally, the increasing prevalence of urinary disorders among the aging population is expected to accelerate catheter adoption, reinforcing APAC’s position as a dynamic and expanding market.
The United States leads the North American market with its well-established healthcare ecosystem and advanced medical device manufacturing sector. The availability of cutting-edge urinary catheter technologies, combined with supportive reimbursement frameworks, fuels strong demand across hospitals, long-term care facilities, and outpatient settings.
China is a major growth driver in the Asia Pacific region, propelled by rising healthcare expenditures, rapid urbanization, and expanding geriatric demographics. Domestic manufacturers are innovating to meet the increasing need for affordable, high-quality urinary catheters.
India’s urinary catheters market is witnessing rapid growth due to improving healthcare infrastructure, increasing government initiatives to enhance urological care, and the rising prevalence of urinary disorders. Growing medical tourism is also a notable contributor to market expansion.
Japan’s aging population and sophisticated healthcare system support steady demand for advanced urinary catheter solutions. The country is investing in research and development, emphasizing minimally invasive and patient-friendly catheter technologies.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 6.19 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.1% | 2032 Value Projection: | USD 9.37 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
B. Braun SE, Coloplast Corp, BD, Cathetrix, Hollister Incorporated, Boston Scientific Corporation, Medtronic, Bactiguard AB, Amecath, Urocare Products, Inc., Rocamed, Cook, Ribbel International Limited, Sonoma Pharmaceuticals, Inc., Affera, Inc., Potrero Medical, Ingenion Medical, and Otsuka Pharmaceutical Factory, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: A urinary catheter is a hollow, partially flexible tube that collects urine from the bladder and leads to a drainage bag. Urinary catheters come in many sizes and types. Catheters are made from rubber and non-rubber materials such as silicone. It is important to use the correct type of catheter and a non-rubber catheter is used if one is allergic to latex. A urinary catheter is used when an individual is having problems urinating naturally. It allows the individual or patient to urinate and drain while having an obstruction in the tube that carries urine out of the bladder (urethra).
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients