The urea breath test is used to detect Helicobacter pylori (H. pylori), a type of bacteria that may infect the stomach and is a main cause of ulcers in both the stomach and duodenum (the first part of the small intestine).
In several countries, assessment studies are conducted for non-invasive 13C urea breath test (UBT) in comparison to other alternative tests such as endoscopy and stool antigen test. For instance, in July 2020, a pilot study was conducted in Alkarak teaching hospital, Jordan for understanding the benefits of UBT over other diagnostic tests for detecting H. pylori. The study concluded 13C UBT was more accurate and sensitive (94.1%) than the stool antigen test and can be a reliable substitute for invasive tests such as endoscopy.
Several studies are conducted for evaluating the efficiency of urea breath test for detection of Helicobacter pylori. For instance, in 2019, a multicenter clinical study was conducted by Exalenz Bioscience Ltd, Israel for analyzing the specificity and sensitivity of BreathID Hp Lab System. There are several advantages offered by the system such as continuous evaluation of breath samples in real-time point-of-care analyses along with providing accuracy of around >99% . Hence, such evaluation studies of urea breath test against histology and rapid urease test are contributing to its high adoption rate during the forecast period.
The global urea breath test market is estimated to be valued at US$ 115.96 million in 2022 and expected to exhibit a CAGR of 5.4% over the forecast period (2022-2030)
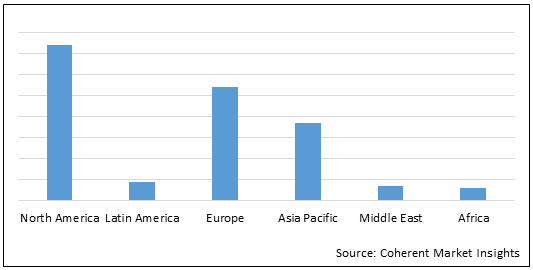
Figure 1. Global Urea Breath Test Market Share (%) in Terms of Value, By Region, 2022
To learn more about this report, Download Free Sample
Increasing studies focused on urea breath test over histopathogical test for H. Pylori detection is expected to witness significant growth of the market in the future
For instance, in June 2021, a research study was published in Dove Press Ltd., which compared diagnostic value for 14C urea breath test in comparison to histopathology in Indonesian dyspeptic patients. The paper highlighted the fact that there is very limited number of hospitals with endoscopy system in Indonesia. Hence, alternative non-invasive methods such as urea breath test will be favorable alternative for covering Indonesian populations for diagnosing H. pylori.
Urea Breath Test Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 115.96 Mn |
| Historical Data for: | 2017 to 2021 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 5.4% | 2030 Value Projection: | US$ 175.97 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Kibion AB (Mayoly Spindler), Avanos Medical, Inc.,Quest Diagnostics, Beijing Richen-Force Science & Technology Co., Ltd., Paladin Labs Inc., Beijing Binal Health Bio- Sci & Tech Co., Ltd., AB ANALITICA s.r.l., Otsuka Holdings Co., Ltd., Sercon Group, Campro Scientific GmbH, Shenzhen Zhonghe Headway Bio-Sci & Tech Co., Ltd., Avisa Diagnostics, INFAI GmbH, Meridian Bioscience, Inc., Metabolic Solutions, Inc., Laboratory Corporation of America Holdings, , Gulf Coast Scientific, Inc., FAN GmbH, and Kizlon Medical |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
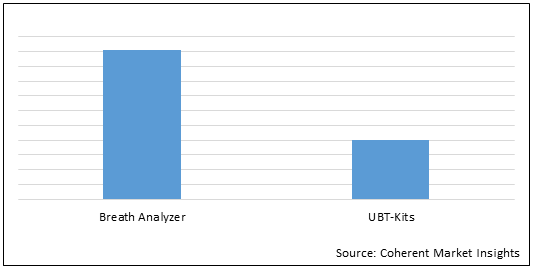
Figure 2. Global Urea Breath Test Market Share (%), by Product, 2022
To learn more about this report, Download Free Sample
Global Urea Breath Test Market: Restraint
There are several diagnostic assays which can be used for detection of H. pylori infection. These are broadly divided into invasive methods and non-invasive methods. The invasive method used for evaluation of H. pylori consists of histopathological examination, molecular testing, and other culture-based tests, while non-invasive techniques includes urea breath test (UBT) and stool antigen test (SAT).
Hence, the presence of several alternative tests other than Urea Breath Test (UBT) for detection of H. pylori infection is a major factor hampering the market growth
In addition, there are certain limitations of UBT which has affected its adoption rate negatively. The following are the two major limitations of UBT:
Key Players
Major players operating in the global urea breath test market include Kibion AB (Mayoly Spindler), Avanos Medical, Inc.,Quest Diagnostics, Beijing Richen-Force Science & Technology Co., Ltd., Paladin Labs Inc., Beijing Binal Health Bio- Sci & Tech Co., Ltd., AB ANALITICA s.r.l., Otsuka Holdings Co., Ltd., Sercon Group, Campro Scientific GmbH, Shenzhen Zhonghe Headway Bio-Sci & Tech Co., Ltd., Avisa Diagnostics, INFAI GmbH, Meridian Bioscience, Inc., Metabolic Solutions, Inc., Laboratory Corporation of America Holdings, x, Gulf Coast Scientific, Inc., FAN GmbH, and Kizlon Medical
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients