Gene therapy can be described as the transfer of genetic material mostly in a vector or carrier while cell therapy includes the transfer of cells with the applicable function into the patient’s body. In the broader sense, gene therapy involves introduction, withdrawal, or modification in genetic code with an aim of curing or treating a disease. Gene therapy can be utilized to minimize the level of a disease-causing form of a protein and enhance the production of disease-fighting proteins, or to produce modified proteins. Cell therapy is the transfer of live, intact cells into a patient’s body in order to cure or treat a disease. The introduced cells can be from patient’s own body or from the donor. The cells utilized in cell therapy can be categorized by their possibilities to transform into different cell types. Cell and gene therapies are widely used to treat severe diseases such as cancer as well as rare diseases such as sickle cell disease, muscular dystrophy, and progeria.
The U.K. cell and gene therapy research challenges market is estimated to be valued at US$ 848 million in 2021 and is expected to exhibit a CAGR of 23.6% over the forecast period (2021-2028).
Figure 1. U.K. Cell and Gene Therapy Research Challenges Market Share (%), By Therapy Type, 2021
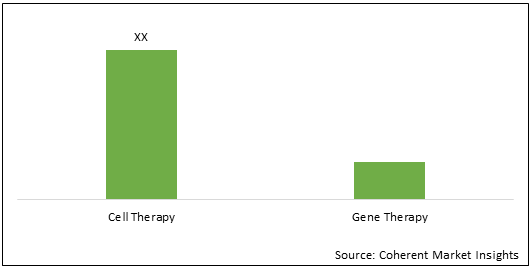
To learn more about this report, Download Free Sample
The increasing product launches and approvals are expected to drive the market growth over the forecast period.
For instance, in October 2018, Bluebird bio, Inc. a biotechnology company, received European Medicines Agency (EMA) acceptance for the company’s authorization application (MAA) for its investigational ‘LentiGlobin gene therapy’. This therapy is used for treating adults and adolescents suffering from transfusion-dependent β-thalassemia (TDT). LentiGlobin gene therapy being studied as a possible treatment to address the significant genetic cause of TDT which may reduce or eliminate the requirement for blood transfusions.
U.K. Cell and Gene Therapy Research Challenges Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2020 | Market Size in 2021: | US$ 848 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2021 to 2028 |
| Forecast Period 2021 to 2028 CAGR: | 23.6% | 2028 Value Projection: | US$ 2525 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Novartis International AG, Pfizer Inc., Sanofi S.A., Amgen, Inc., PTC Therapeutics, Inc., Orchard Therapeutics Plc., Biomarin Pharmaceutical Inc., Regeneron, Bluebird Bio, Inc. (Celgene Corporation), Freeline Therapeutics Ltd, MeiraGTx Limited, Kolon TissueGene, Inc., Kite Pharma, Inc. (Gilead Sciences, Inc.), Biogen INC, and Bristol-Myers Squibb Company |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2. U.K. Cell and Gene Therapy Research Challenges Market Share (%), By Indication, 2021
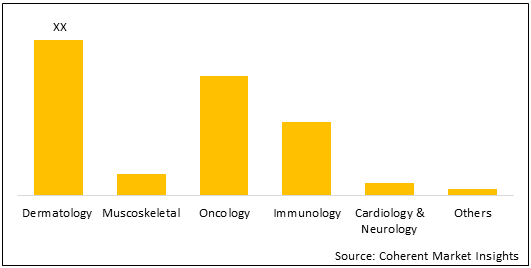
To learn more about this report, Download Free Sample
The increasing adoption of inorganic growth strategies such as mergers, collaborations, partnerships, and acquisitions by key players in order to strengthen their position in the U.K. market is expected to drive the market growth over the forecast period.
For instance, in March 2019, Biogen Inc., pioneers in neuroscience entered into an agreement to acquire Nightstar Therapeutics, a clinical-stage gene therapy company, to set up a clinical pipeline of gene therapy candidates in the field of ophthalmology.
U.K. Cell and Gene Therapy Research Challenges Market – Impact of Coronavirus (COVID-19) Pandemic
The COVID-19 pandemic and lockdown across the globe has negatively impacted the financial status of businesses across all sectors. The COVID-19 pandemic has impacted the entire supply chain of the healthcare industry mainly due to strict lockdown in several regions. Likewise, COVID-19 pandemic has made significant impact on the UK cell and gene therapy market.
The cell and gene therapy development activities had slowed down in pandemic as companies had switched focus to vaccine production. The regulatory guidelines for advanced therapy medicinal products (ATMPs) is complex, with complexities in reimbursement environment, regulatory regime, and supply chain. There are issues in conduction of trials for small patient population suffering through rare diseases. These complications have increased due to COVID-19 pandemic.
U.K. Cell and Gene Therapy Research Challenges Market - Restraint
Unfavourable reimbursement policies and manufacturing issues limiting the availability of products at treatment centres are expected to hinder growth of the U.K. cell and gene therapy research challenges market over the forecast period. However, cell and gene therapy has witnessed multiple setbacks from regulatory authorities in charge of reimbursement policy, which is expected to inhibit growth of the market.
For instance, in August 2018, the National Institute for Health and Care Excellence (NICE), U.K. concluded that Yescarta is a very expensive therapy and hence cannot be considered as a cost-effective use of National Health System (NHS) resources.
Key Players
Major players operating in the U.K. cell and gene therapy research challenges market include Novartis International AG, Pfizer, Inc., Sanofi S.A., Amgen, Inc., PTC Therapeutics, Inc., Orchard Therapeutics Plc., Biomarin Pharmaceutical Inc., Regeneron, Bluebird Bio, Inc. (Celgene Corporation), Freeline Therapeutics Ltd, MeiraGTx Limited, Kolon TissueGene, Inc., Kite Pharma, Inc. (Gilead Sciences, Inc.), Biogen INC., and Bristol-Myers Squibb Company.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients