UAE Heart Failure Treatment Drugs Market is estimated to be valued at USD 90.7 Mn in 2025 and is expected to reach USD 140.9 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 6.5% from 2025 to 2032.
Analysts’ Views on the UAE heart failure treatment drugs market:
The increasing prevalence of heart diseases, new product launches, and strategies like mergers, acquisitions, and collaborations are expected drive the UAE heart failure treatment drugs market growth over the forecast period. For instance, according to data published by British Medical Journal Cardiovascular Disorders on March 15, 2023, Cardiovascular Disease (CVD) is the leading cause of death in the world. In the United Arab Emirates (UAE), it accounts for 40% of mortality. CVD is caused by multiple Cardiometabolic Risk Factors (CRFs) including obesity, dysglycemia, dyslipidemia, hypertension, and central obesity. Such high rate of mortality due to heart-related disorders can drive the growth of the UAE heart failure treatment drugs market.
UAE Heart Failure Treatment Drugs Market - Drivers
Increasing research and development activities in heart failure treatment
Increasing research and development activities in heart failure treatment are expected to drive the UAE heart failure treatment drugs market growth over the forecast period. For instance, according to an article published by Journal of Cardiac Failure in August 2022, a study was conducted across different locations including the UAE to assess the clinical integration and safety of the HeartLogic multisensor index and alerts in Heart Failure (HF) care. The multiple cardiac sensors for management of heart failure (MANAGE-HF) study enrolled 200 patients with HF and reduced ejection fraction. Mean age of participants was 67 years. 61% had HF hospitalization in prior 12 months. During follow up, there were 585 alert cases with average 1.76 alert cases per patient year. HF medications were augmented during 74% of the alert cases. HF treatment augmentation within 2 weeks from an initial alert was associated with more rapid recovery of the HeartLogic Index. It was concluded that HeartLogic alert management was safely implemented in HF care and may optimize HF management.
Increasing launch of new treatment methods
Increasing launch of new treatment therapeutics is expected to drive the UAE heart failure treatment drugs market growth. For instance, on May 6, 2020, AstraZeneca, a U.K.-based multinational pharmaceutical and biotechnology company, announced the launch of Farxiga (dapagliflozin) to reduce the risk of Cardiovascular (CV) death and hospitalization for heart failure in adults with reduced ejection fraction (HFrEF) or with and without Type-2 Diabetes (T2D). The approval was based on positive results from the landmark Phase III DAPA-HF trial, which showed Farxiga achieving a statistically significant and clinically meaningful reduction of CV death or hospitalization for Heart Failure (HF), compared to placebo. Farxiga is the first Sodium Glucose Co-transporter 2 (SGLT2) inhibitor approved by the U.S. Food and Drug Administration indicated to treat patients with HFrEF.
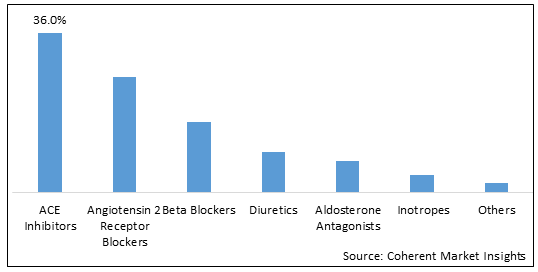
Figure 1. UAE Heart Failure Treatment Drugs Market Share (%), By Drug Class, 2025
To learn more about this report, Download Free Sample
UAE Heart Failure Treatment Drugs Market - Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 affected the economy in three main ways: by directly affecting the production and demand of drugs, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries, such as China, India, Saudi Arabia, the U.A.E., Egypt, and others faced problems regarding the transportation of drugs from one place to another.
However, the COVID-19 pandemic had a negative impact on the UAE heart failure treatment drugs market due to nationwide lockdowns. For instance, according to an article published by Heart Views on October 13, 2020, there were some challenges in heart failure care during the pandemic situation. This is because of diagnostic delays, misdiagnosis of COVID-19 in patients with HF due to overlapping symptoms, reduced/disrupted follow up, reduced/no face-to-face contact with clinicians, and difficulty obtaining medication. This reduced the quality of treatment given to heart failure patients in UAE.
UAE Heart Failure Treatment Drugs Market Segmentation:
The UAE heart failure treatment drugs market report is segmented into drug class and distribution channel.
Based on drug class, the UAE heart failure treatment drugs market is segmented into ACE inhibitors, angiotensin 2 receptor blockers, beta blockers, diuretics, aldosterone antagonists, inotropes, and others. Out of which, the ACE inhibitors segment is expected to dominate the market due to increasing launch of products.
Based on distribution channel, the UAE heart failure treatment drugs market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. Among these, the retail pharmacies segment is expected to dominate the market over the forecast period due to the increasing availability of cardiac failure drugs in retail pharmacy chains.
Among all segmentation, the drug class segment has the highest potential due to the increasing launch of products by the key market players. For instance, according to an article published by American Heart Association on April 29, 2020, a study was conducted to understand effect of ACE inhibitors on virus infection, inflammatory status, and clinical outcomes in patients with COVID infection. It was concluded that without adding risk for COVID 19 infection, the ACE inhibitors treatment is superior to other hypertensive treatment in reducing high sensitive C-reactive protein and procacitonin levels in patients with COVID-19 and preexisting hypertension.
UAE Heart Failure Treatment Drugs Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 90.7 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.5% | 2032 Value Projection: | USD 140.9 Mn |
| Segments covered: |
|
||
| Companies covered: |
Novartis International AG, AstraZeneca Plc., Amgen, Inc., Pfizer, Inc., Mylan N.V., Merck & Co., Inc., Bristol-Myers Squibb, GlaxoSmithKline Plc., Teva Pharmaceutical Industries Ltd., and Bayer AG |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
UAE Heart Failure Treatment Drugs Market: Key Developments
On January 20, 2021, Merck & Co., Inc., a U.S.-based multinational pharmaceutical company, announced the launch of Verquvo, a soluble guanylate cyclase to reduce risk of cardiovascular death and heart failure hospitalization following hospitalization for heart failure or need for outpatient intravenous diuretics in adults with symptomatic heart failure and ejection fraction less than 45%. The approval is based on the results of pivotal phase 3 victoria trials. Trials were conducted on 5,050 adult patients with worsening heart failure event. A worsening heart failure event was defined as heart failure hospitalization within six months or less prior to randomization or use of outpatient IV diuretics for heart failure within three months or less prior to randomization. In VICTORIA, the primary endpoint was a composite of time to first event of cardiovascular death or hospitalization for heart failure. The median follow-up for the primary endpoint was 11 months. VERQUVO was superior to placebo in reducing the risk of cardiovascular death or heart failure hospitalization based on a time-to-event analysis.
On February 4, 2022, Cytokinetics Incorporated U.S. based biopharmaceutical company announced the acceptance of New Drug Application (NDA) for Omecamtiv Mecarbil, an investigational, selective, and small molecule cardiac myosin activator for the treatment of heart failure with reduced ejection fraction. The NDA is supported by results from global approach to lowering adverse cardiac outcomes through improving contractility in heart failure (GALACTIC-HF) phase 3 cardiovascular outcomes clinical trials of omecamtiv mecarbil that enrolled 8,000 patients in 35 countries across 945 sites. GALACTIC-HF demonstrated a statistically significant effect of treatment with omecamtiv mecarbil to reduce the risk of the primary composite endpoint of Cardiovascular (CV) death or heart failure events (heart failure hospitalization and other urgent treatment for heart failure) compared to placebo in patients treated with standard of care. Additional analyses from GALACTIC-HF demonstrated a greater treatment effect of omecamtiv mecarbil in patients with lower Left Ventricular Ejection Fraction (LVEF), as well as other characteristics that may indicate worsening heart failure.
On June 14, 2023, the UAE joined forces with health authorities in Syria to carry out crucial heart operations in underprivileged communities. The Cardiac Care initiative will be carried out by Emirates Red Crescent, UAE affiliate of the International Federation of Red Cross and Red Crescent Societies in conjunction with the Health Directorate in the Latakia governorate, in North-Western Syria, Al Bassel Cardiology Hospital in Latakia and a specialist medical team. The project will offer catheterization and heart operations, and help address concerns over the prevalence of heart disease in the area.
On April 26, 2022, RAK Hospital, part of Arabian Healthcare Group, a UAE-based private hospital, introduced a new cardiac defibrillator therapy that can save patients on the verge of a heart failure. A CRT-D is a special device for patients who are at a high risk of a sudden cardiac arrest. While functioning like a normal pacemaker to treat slow heart rhythms, a CRT-D device also delivers small electrical impulses to the left and right ventricles to help them contract at the same time. This helps the heart pump more efficiently.
UAE Heart Failure Treatment Drugs Market: Key Trends
Launch of new heart failure medications
The introduction of newer heart failure medications can drive the market growth. On May 30, 2023, Sotagliflozin was launched by Lexicon Pharmaceuticals, Inc., U.S. -based biopharmaceutical company developing treatments for human disease. It is once a day pill with brand name Inpefa. The launch is based on the SCORED and SOLOIST-WHF trials, showing reductions in HF hospitalizations in diabetic patients with chronic kidney disease and in decompensated HF, respectively, with the benefits emerging after just a few months. Additional analyses confirmed a benefit across ejection fractions, including HF with preserved ejection fraction (HFpEF). The drug enters a market already dominated by the Sodium-glucose Cotransporter 2 (SGLT2) inhibitors empagliflozin and dapagliflozin both of which now have labeled indications across the spectrum of LVEF. Of note, sotagliflozin is actually a dual inhibitor of SGLT1, the primary transporter for glucose absorption in the gut, and SGLT2, which reabsorbs glucose in the kidney.
Awareness programs by key market players
Awareness programs by key market players can drive the UAE heart failure treatment drugs market growth over the forecast period. For instance, on November 14, 2022, Novartis AG, a Switzerland-based global healthcare company, partnered with Emirates Cardiac Society (ECS) for a campaign that seeks to raise awareness around heart failure in the U.A.E. The campaign is called ‘Your Heart Can’t Wait’ to educate residents across the emirates about the symptoms of heart failure, and how delaying diagnosis and treatment puts a patient at risk of recurrent hospitalizations that can be fatal. Several leading healthcare providers in the UAE have been engaged to ensure that patients have access to the right information and are empowered to make the right decisions regarding their treatment. The experts shared a proper cardiac evaluation, along with some simple lifestyle changes that can help in improving the heart health. They have also shared a strong correlation between factors such as obesity, smoking, high blood pressure, and diabetes.  
UAE Heart Failure Treatment Drugs Market: Restraints
Strict rules and regulations in the UAE
The strict rules and regulations in the UAE are expected to hamper the UAE heart failure treatment drugs market growth. For instance, according to an article published in Handbook of Healthcare in the Arab World on August 11, 2021, pharmaceutical regulations and guidelines are stricter in Middle Eastern countries, such as UAE, Saudi Arabia, and others, compared to other countries across the world. Most Middle Eastern pharmaceutical legislation details are not accessible to the public and private pharmaceutical companies across the globe. Moreover, the legislation is only available in the local language, making comprehension difficult for multinational pharmaceutical companies to launch pharmaceutical projects in Middle Eastern countries.
To counterbalance this restraint, more user friendly guidelines should be introduced.
Limited availability of pharmaceutical products across Middle East countries
Limited availability of pharmaceutical products across Middle East countries is expected to hamper the UAE heart failure treatment drugs market growth. For instance, according to an article published in Journal of Pharmaceutical Policy and Practice on December 16, 2021, pharmaceutical players across the globe have registered fewer products in the Middle Eastern countries, such as UAE, Saudi Arabia, and others, which may result in the limited availability of medicinal products in these countries. The number of pharmaceutical companies available in the UAE are only 23. Such slow growth of the pharmaceutical sector can hamper the market growth.
To counterbalance this restrain, more investments in the Middle East countries should be done for the development and availability of pharmaceutical products.
UAE Heart Failure Treatment Drugs Market - Key Players
The major players operating in the UAE heart failure treatment drugs market include Novartis International AG, AstraZeneca Plc., Amgen, Inc., Pfizer, Inc., Mylan N.V., Merck & Co., Inc., Bristol-Myers Squibb, GlaxoSmithKline Plc., Teva Pharmaceutical Industries Ltd., and Bayer AG.
Definition:Heart failure is characterized by the inability of the heart to pump adequate amount of blood to the body. There are three types of heart failures: left-sided heart failure, right-sided heart failure, and biventricular heart failure. The condition is treated with particular cardiovascular drugs available in the market. Moreover, the treatment for heart failure depends on the stages (stage A, B, C, & D). Drugs such as diuretics, ACE inhibitors, angiotensin 2 receptor blockers, beta blockers, and digitalis are majorly used in the treatment of heart failure.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients