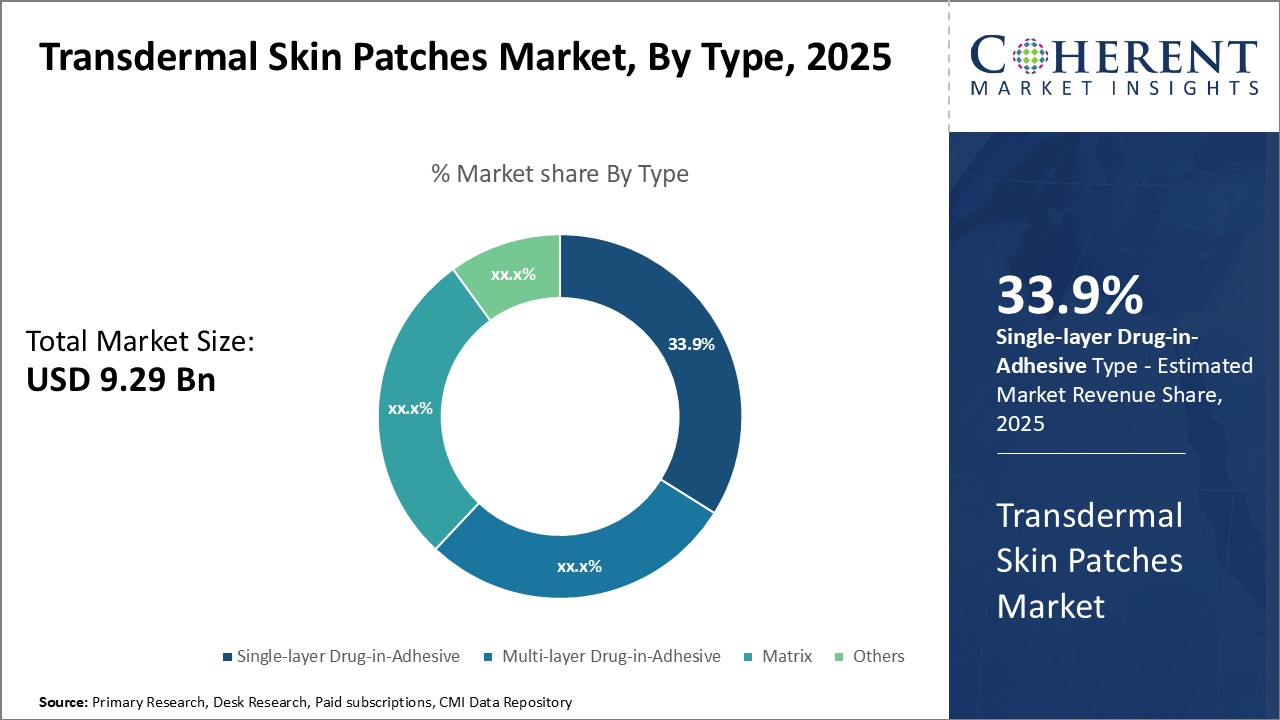
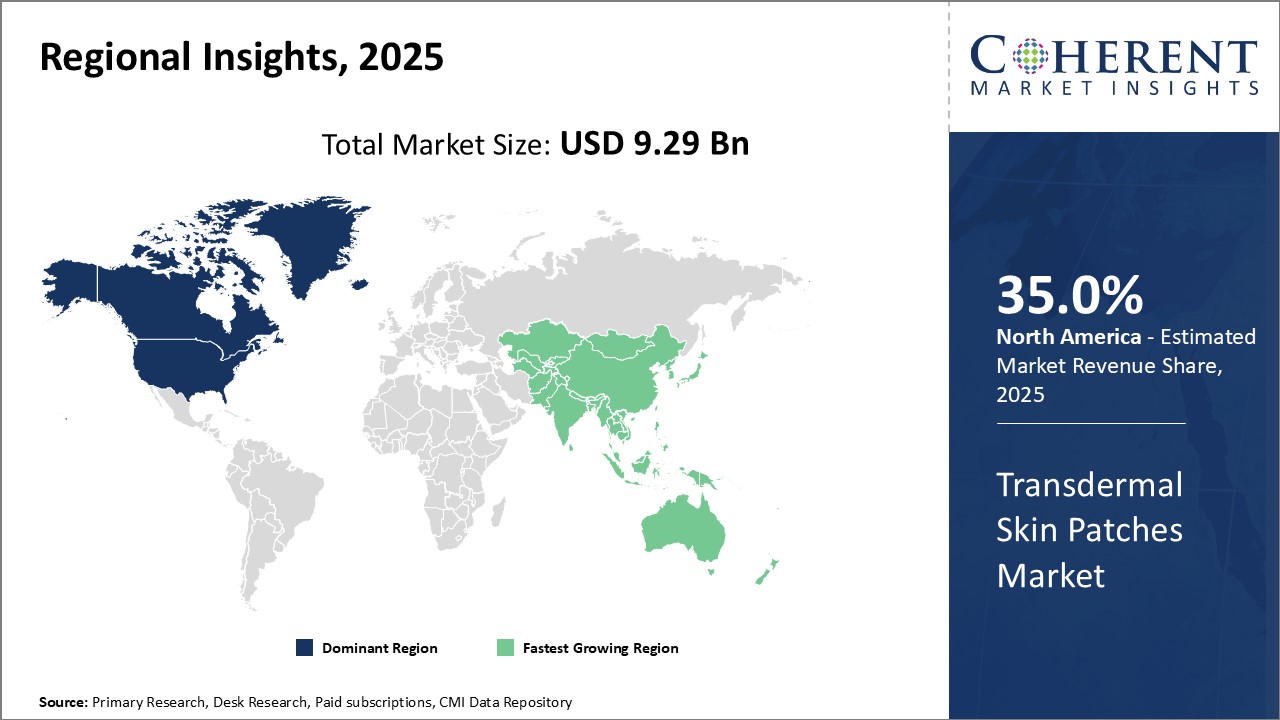
The global transdermal skin patches market is estimated to be valued at USD 9.29 Bn in 2025 and is expected to reach USD 13.71 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 5.7% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The global transdermal skin patches market is witnessing increasing demand for advanced transdermal patches that can deliver both small and large molecule drugs for extended periods. The rising popularity of wearable patches is another key trend in the market. Technological advancements to incorporate features like sensors and wireless connectivity into skin patches are expanding their applications beyond healthcare into sectors like consumer goods and fitness.
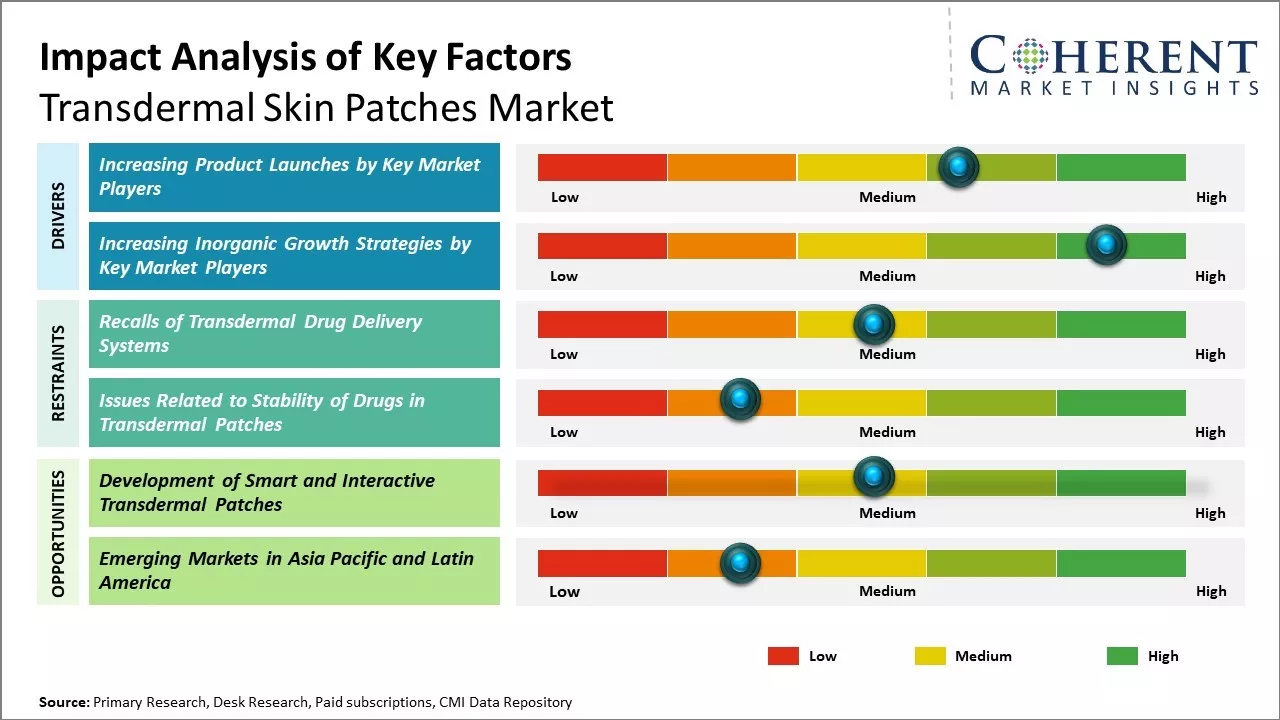
Increasing Product Launches by Key Market Players
Increasing adoption of organic growth strategies, such as product launches by key market players, is expected to drive the market growth over the forecast period. For instance, in September 2022, Corium, Inc., a pharmaceutical company, announced that it had launched Adlarity, a transdermal patch for treating Alzheimer’s disease. Adlarity, a once-weekly patch, delivers consistent doses of donepezil through the skin. It treats patients with mild, moderate, or severe dementia of the Alzheimer’s type.
Get actionable strategies to beat competition: Download Free Sample
Increasing Inorganic Growth Strategies by Key Market PlayersIncreasing adoption of inorganic growth strategies, such as agreements, is expected to drive the market growth over the forecast period. For instance, in March 2023, BioNxt Solutions Inc., a pharmaceutical company, announced that it has executed an agreement to carry out its comparative drug absorption study for the company’s transdermal (“TDS”) Rotigotine patch for the treatment of Parkinson’s disease.

To learn more about this report, Download Free Sample
Market Challenges – Recalls of Transdermal Drug Delivery SystemsDrug failure and recalls of transdermal drug delivery systems is expected to hamper the growth of the transdermal skin patches market over the forecast period. For instance, in November 2021, Teva Pharmaceutical Industries Ltd., a pharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) recalled a single lot of clonidine transdermal patches for failing to meet impurities/degradation specifications.
Market Opportunities – Development of Smart and Interactive Transdermal Patches
The development of smart and interactive transdermal patches offers promising opportunities for growth in the global transdermal skin patches market. These next-generation patches go beyond basic transdermal drug delivery by incorporating advanced technologies that allow for real-time patient monitoring and feedback. Smart transdermal patches are embedded with miniaturized sensors, electronics, and communication capabilities. This allows them to passively collect various biomarker data from the skin, such as temperature, hydration levels, glucose concentrations, and others. The collected data can provide medical insights in real-time and be transmitted wirelessly to doctors or caregivers. For patients with chronic conditions like diabetes and cardiac disorders, this added level of round-the-clock monitoring can help manage their care more proactively and prevent critical complications.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
Insights, By Type: Convenient Administration Drives Single-layer Drug-in-AdhesiveThe type segment includes single-layer drug-in-adhesive, multi-layer drug-in-adhesive, matrix, and others. The single-layer drug-in-adhesive sub-segment is estimated to hold 33.9% of the market share in 2025 owing to its convenient mode of administration. This segment consists of a single layer of backing layer integrated with drug reservoir or matrix. Due to its simple structure and presence of drug in a single layer, single-layer patches provide ease of administration for patients. The drug from the reservoir or matrix diffuses directly through the skin at a predetermined rate. This ensures steady release of drug into the bloodstream over an extended time period. The single step administration process without involvement of additional adhesive layers makes this type of patch very convenient to use. Patients find it easy to apply these patches on the skin in comparison to multilayer patches. This enhances compliance for long-term treatment protocols. The consistent drug delivery with minimal fluctuations also provides effective therapeutic outcomes. Lack of multiple interfaces for drug to permeate through also improves bioavailability for some drugs. All these factors contribute to higher preference and uptake of single-layer drug-in-adhesive patches.
Insights, By Application: Pain Relief Fuels Demand in Pain Management
The application segment includes pain relief, smoking cessation, overactive bladder, hormonal therapy, and others. The pain relief sub-segment is estimated to hold 32.4% of the market share in 2025. Transdermal patches play a vital role in effective pain management. They help deliver analgesic drugs such as fentanyl and buprenorphine directly into the bloodstream through the skin. This ensures steadier drug levels for long-lasting relief from various types of moderate to severe acute and chronic pain conditions. Transdermal patches minimize fluctuations associated with oral pain medications. This provides more consistent pain relief without sharp peaks and troughs in drug levels. The non-invasive nature of patches enhances comfort for patients, especially those unable to swallow oral medications. Transdermal administration also avoids issues like gastrointestinal irritation caused by oral analgesics. All these advantages have fueled the widespread use of patches for relief from various cancer, postoperative, musculoskeletal, and neuropathic pain types. New patches containing combinations of opioids and non-opioids are further extending indications.
Insights, By Distribution Channel: Accessibility Augments Hospital Pharmacies' Position
The distribution channel segment includes hospital pharmacies, retail pharmacies, and online pharmacies. The hospital pharmacies sub-segment is estimated to hold 34.7% of the market share in 2025 owing to their widespread accessibility and matchless convenience. Being an integral part of hospitals, these pharmacies allow the seamless procurement of transdermal patches for inpatients receiving long-term treatments. They also help caregivers obtain patches for patients visiting hospitals regularly for therapies or surgeries. Located within the healthcare premises, hospital pharmacies offer the highest level of accessibility anytime without additional travel. This proves extremely convenient, especially for patients confined to beds or with limited mobility. The pharmacists' expertise helps address medication queries and ensures proper guidance on patch applications. Low inventory costs and bulk procurement abilities of hospitals further enable subsidized supply through their in-house pharmacies. All these factors drive a notable preference for hospital pharmacies as the most approachable distribution nodes.
Need a Different Region or Segment? Download Free Sample
North America remains the dominant region in the global transdermal skin patches market and is estimated to hold 35.0% of the market share in 2025. This due to a well-established healthcare infrastructure and higher adoption of innovative drug delivery technologies. Major pharmaceutical companies have their headquarters in the U.S. and they are continuously investing in R&D to develop new transdermal patches. Due to stringent U.S. Food and Drug Administration approval process, only high-quality patches are available in the market. Furthermore, awareness regarding benefits of transdermal drugs over oral medications is significantly high among consumers as well as physicians.
The Asia Pacific region, on the other hand, is anticipated to witness the fastest growth over the forecast period. Rapidly developing economies like India and China are expected to be the key contributors. Rising healthcare expenditure and increasing focus of international players to tap opportunities in emerging nations will drive the market in Asia Pacific. India, in particular, stands out with its generic drugs industry. Several local manufacturers in India are engaged in production of cost-effective transdermal patches for pain, hormone replacement, and nicotine cessation therapies. This makes transdermal drugs accessible to masses.
Transdermal Skin Patches Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 9.29 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.7% | 2032 Value Projection: | USD 13.71 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
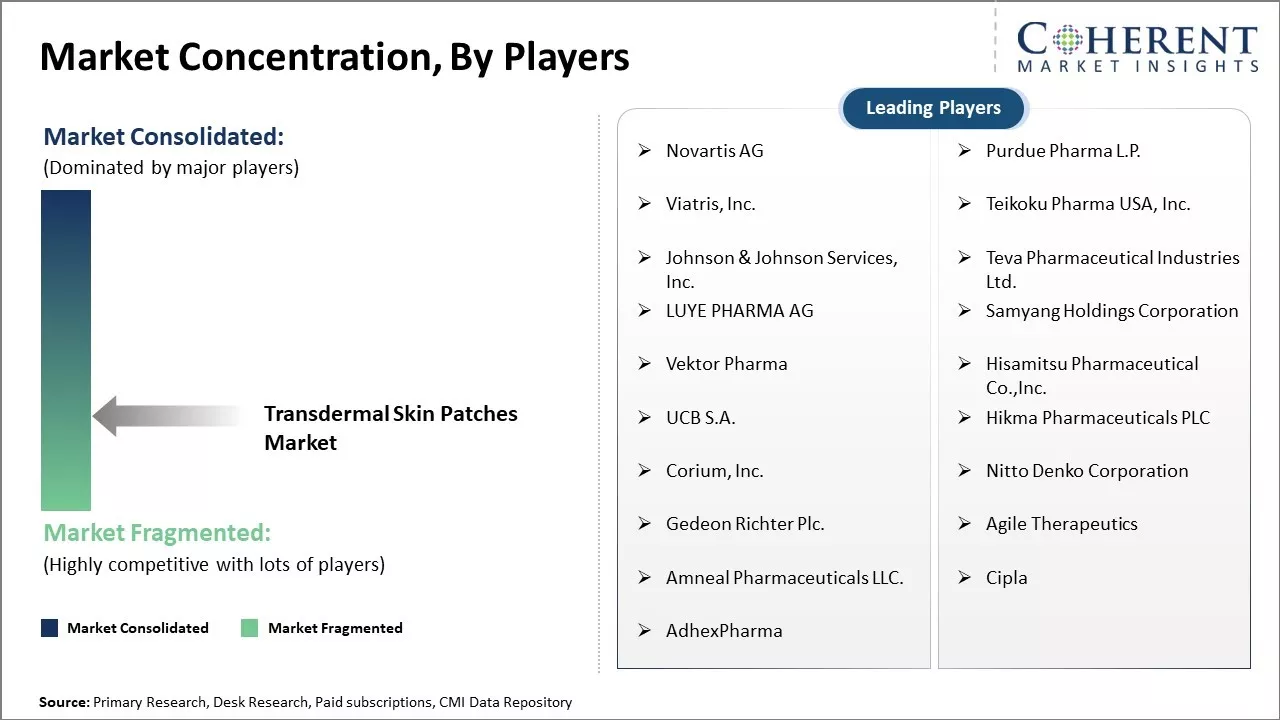
| Companies covered: |
Novartis AG, Purdue Pharma L.P., Viatris, Inc., Teikoku Pharma USA, Inc., Johnson & Johnson Services, Inc., Teva Pharmaceutical Industries Ltd., LUYE PHARMA AG, Samyang Holdings Corporation, Vektor Pharma, Hisamitsu Pharmaceutical Co.,Inc., UCB S.A., Hikma Pharmaceuticals PLC, Corium, Inc., Nitto Denko Corporation, Gedeon Richter Plc., Agile Therapeutics, Amneal Pharmaceuticals LLC., Cipla, and AdhexPharma |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients