Transcatheter Mitral Valve Repair And Replacement Market Size and Forecast – 2025 – 2032
The Global Transcatheter Mitral Valve Repair and Replacement Market size is estimated to be valued at USD 1.8 billion in 2025 and is expected to reach USD 5.7 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 16.2% from 2025 to 2032.
Global Transcatheter Mitral Valve Repair And Replacement Market Overview
Transcatheter mitral valve repair and replacement (TMVR) systems are advanced minimally invasive cardiac devices designed to treat mitral regurgitation and other valve dysfunctions without the need for open-heart surgery. These products consist of catheter-based delivery systems that allow valve repair or replacement through femoral or transapical access routes. Devices such as clip-based repair systems, annuloplasty rings, and transcatheter prosthetic valves have transformed patient management, particularly for high-risk surgical candidates. Continuous improvements in imaging guidance, 3D echocardiography, and fluoroscopy-assisted delivery have significantly enhanced procedural accuracy and safety.
Key Takeaways
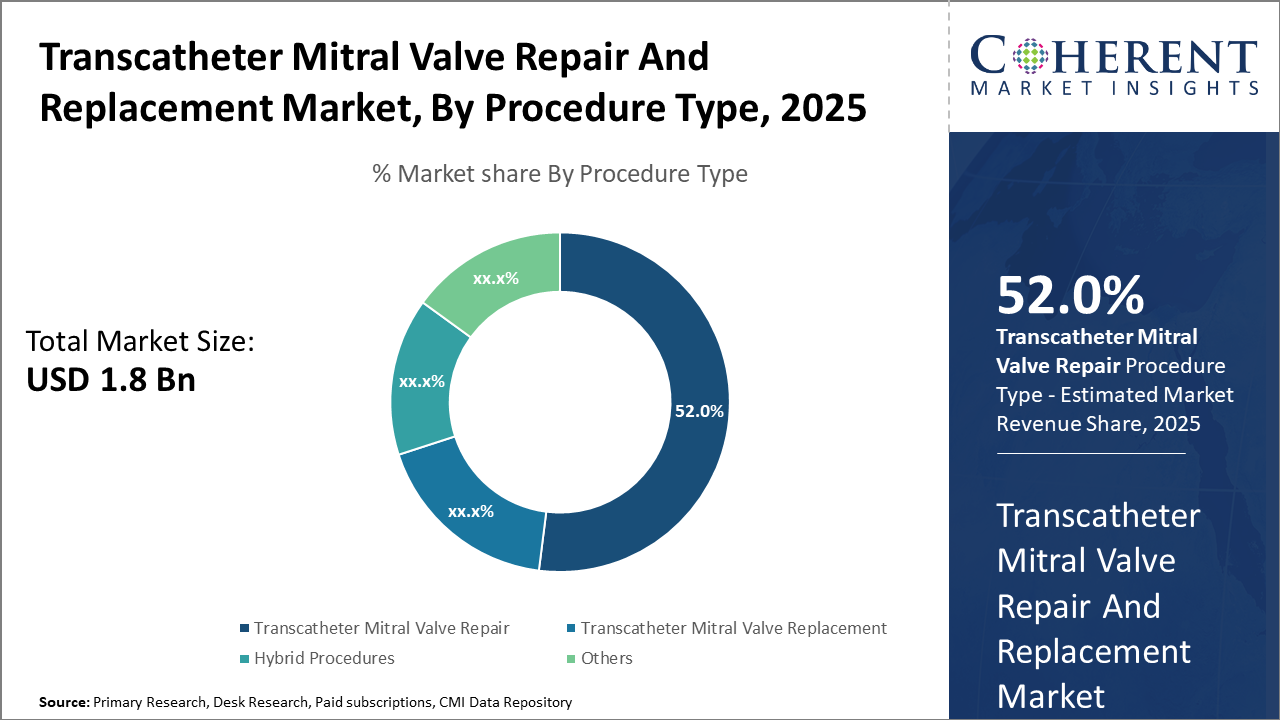
The Transcatheter Mitral Valve Repair segment holds a dominant market share of 52%, primarily due to its proven clinical efficacy and lower procedural risks. Innovations in catheter design are expected to sustain this leadership.
The MitraClip device leads the device type segment, supported by over 60% industry share due to its early market entry and robust clinical outcomes. Emerging replacements like Tendyne show promising growth trajectories.
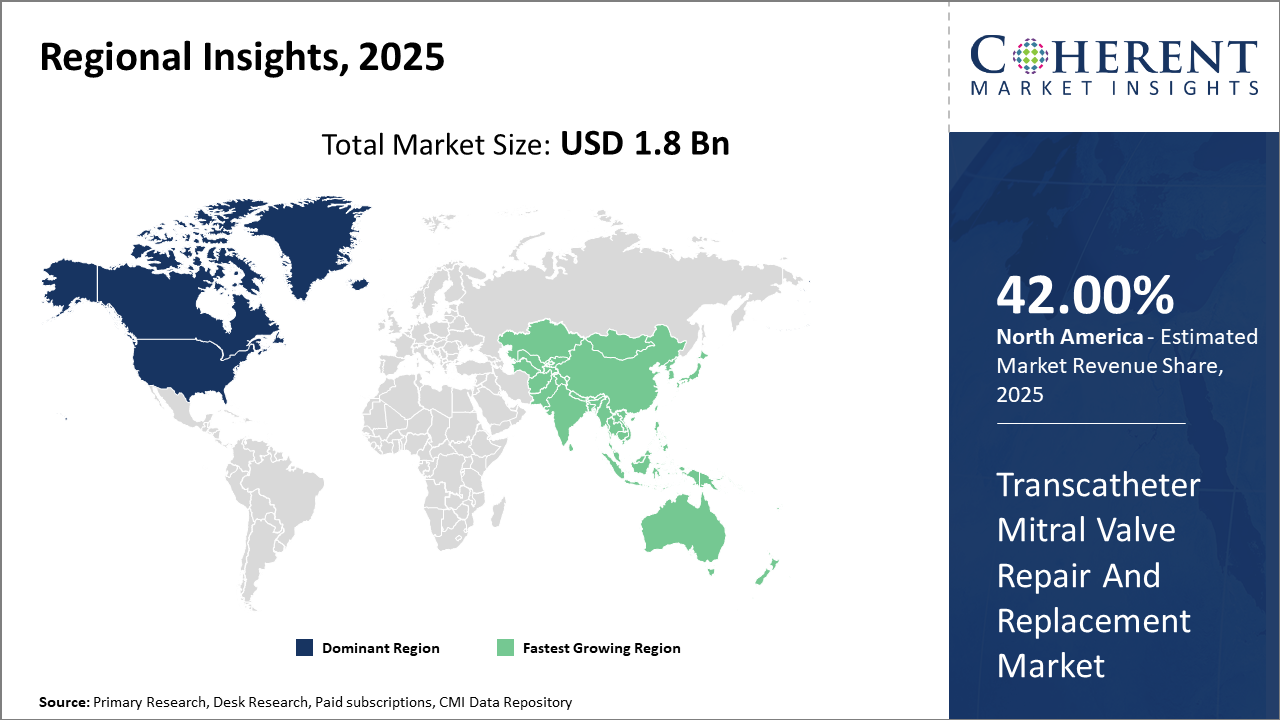
North America dominates the market owing to advanced healthcare infrastructure and high reimbursement rates, accounting for approximately 42% of the transcatheter mitral valve repair and replacement market size.
Asia Pacific represents the fastest growth region driven by increasing disease burden, expansion of healthcare services, and evolving regulatory landscapes, with a CAGR exceeding 18% during the forecast period.
Transcatheter Mitral Valve Repair And Replacement Market Segmentation Analysis
To learn more about this report, Download Free Sample
Transcatheter Mitral Valve Repair And Replacement Market Insights, By Procedure Type
Transcatheter Mitral Valve Repair dominates the market share with 52%, owing to its minimally invasive nature and proven clinical outcomes that reduce hospitalization periods. This segment has capitalized on widespread adoption and reimbursement favorability across mature markets. Transcatheter Mitral Valve Replacement is the fastest-growing subsegment, offering comprehensive solutions for patients unsuitable for repair, driven by innovations such as fully repositionable valves enhancing safety profiles. Hybrid procedures, combining repair and replacement techniques, are gaining niche traction by offering tailored treatment strategies.
Transcatheter Mitral Valve Repair And Replacement Market Insights, By Device Type
MitraClip commands a dominant industry share exceeding 60%, primarily due to its pioneering role and extensive clinical validation supporting widespread adoption. MitraClip devices continue to evolve with enhanced clip sizes and steering mechanisms to improve procedural applicability. The Cardioband system is witnessing rapid growth as the fastest-growing subsegment, propelled by its annulus reduction technique that benefits patients with functional mitral regurgitation. Pascal and Tendyne devices are emerging players featuring advanced anchoring and full valve replacement designs, respectively, challenging traditional repair dominance.
Transcatheter Mitral Valve Repair And Replacement Market Insights, By End-User
Hospitals dominate, holding the largest market share, driven by comprehensive cardiac facilities and the availability of experienced interventional cardiologists equipped to handle complex TMVR cases. Ambulatory surgical centers are the fastest-growing subsegment, leveraging outpatient procedural capabilities and enhanced patient throughput, reflecting evolving healthcare delivery trends. Specialty clinics focus on niche patient groups with tailored therapeutic interventions, contributing to steady market expansion.
Transcatheter Mitral Valve Repair And Replacement Market Trends
The Transcatheter Mitral Valve Repair and Replacement market demonstrates a transition towards fully implantable valve platforms that mitigate paravalvular leak risks observed with repair devices.
For example, in 2024, over 30% of TMVR procedures in Europe involved replacement options, up from 22% in 2023, reflecting growing clinical confidence.
Additionally, deployment of AI-assisted imaging tools for valve sizing and placement has been shown to reduce procedure duration by up to 15%, as evidenced by clinical audits in major U.S. heart centers.
The ongoing integration of hybrid procedures combining repair and replacement techniques is also reshaping treatment paradigms, enabling precision medicine approaches tailored to patient-specific mitral pathology.
Transcatheter Mitral Valve Repair And Replacement Market Insights By Geography
To learn more about this report, Download Free Sample
North America Transcatheter Mitral Valve Repair And Replacement Market Analysis and Trends
In North America, the dominance in the Transcatheter Mitral Valve Repair and Replacement market is driven by superior healthcare infrastructure, early adoption of cutting-edge technologies, and favorable reimbursement mechanisms, accounting for approximately 42% industry share. The presence of several leading market companies and large-scale clinical studies further enhances market revenue and innovation.
Asia Pacific Transcatheter Mitral Valve Repair And Replacement Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth, with an estimated CAGR of over 18%, catalyzed by the rising incidence of valvular heart diseases, government initiatives to expand healthcare access, and increasing medical device approvals. India and China are pivotal drivers, supported by growing healthcare expenditure and localized manufacturing expansions.
Transcatheter Mitral Valve Repair And Replacement Market Outlook for Key Countries
USA Transcatheter Mitral Valve Repair And Replacement Market Analysis and Trends
The USA’s market for transcatheter mitral valve repair and replacement continues to lead globally due to advanced cardiac intervention programs and the presence of major manufacturers. The adoption rate of TMVR procedures increased by 20% in US hospitals between 2023 and 2024, supported by expanded reimbursement coverage under CMS. Leading players have established extensive physician training networks and partnerships with reputable cardiac centers to optimize clinical outcomes and real-world data generation. Additionally, regulatory bodies have accelerated approvals for novel devices following successful multicenter trials. These factors contribute to robust market growth and sustained innovation.
Germany Transcatheter Mitral Valve Repair And Replacement Market Analysis and Trends
Germany’s TMVR market is characterized by high patient awareness and an established network of specialized cardiovascular clinics. The country reported a 15% rise in procedures from 2023 to 2024, driven by favorable insurance policies and reimbursement pathways. Local innovative startups, along with established global entities, contribute to the diverse device ecosystem. Research institutions in Germany are pioneering the integration of next-generation imaging and navigation tools, bolstering the country’s position as a significant market player in Europe. Its strategic location and active participation in European regulatory harmonization further strengthen business growth prospects.
Analyst Opinion
Demand for TMVR devices has surged remarkably due to rising incidences of mitral regurgitation in elderly populations, with over 10 million cases estimated globally in 2024 alone. The demand-side dynamics also reflect widening indications, such as secondary mitral regurgitation, enhancing the overall market size.
Pricing strategies among major market players emphasize value-based models that align with reimbursement trends in major healthcare systems like Medicare in the USA, contributing to higher use rates. For instance, transcatheter mitral valve repair procedures in the US increased by 18% from 2023 to 2024.
Production capacity expansions, notably in leading medical device clusters in the U.S. and Europe, have accelerated market revenue growth by reducing lead times for new device launches. One instance saw a notable 20% output increase by a key manufacturer in early 2024, which helped stabilize supply amid fluctuating demand.
Import and export flows are increasingly influenced by the Asia Pacific’s evolving regulatory frameworks, with countries like Japan fast-tracking device approvals, amplifying regional import volumes by approximately 22% in 2024 compared to 2023.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: |
USD 1.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 16.2% | 2032 Value Projection: |
USD 5.7 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Abbott Laboratories, Edwards Lifesciences Corporation, Medtronic plc, Boston Scientific Corporation, LivaNova PLC, Neochord, Inc., 4Tech Cardio, Tendyne Holdings, Inc., Pi-Cardia, Inc., Valtech Cardio, Cardiovalve Ltd. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Transcatheter Mitral Valve Repair And Replacement Market Growth Factors
The aging population globally remains the foremost driver, with individuals aged 65 and above growing by over 2% annually, thus significantly enlarging the patient pool for TMVR procedures. Additionally, technology advancements such as improved device durability and navigational systems have boosted procedural success rates to over 95% in recent clinical studies from 2024. Reimbursement improvements in established healthcare markets, particularly North America and Europe, are expanding accessibility and hospital adoption. Furthermore, increasing awareness and screening programs for valvular diseases in emerging economies contribute to broader market penetration and revenue growth.
Transcatheter Mitral Valve Repair And Replacement Market Development
In June 2025, Meril Life Sciences launched the MyClip™ Transcatheter Edge-to-Edge Repair (TEER) System, India’s first domestically developed TEER device for the treatment of severe mitral regurgitation (MR) in patients who are high-risk for surgery. The system features a low-profile, repositionable, and retrievable clip delivery mechanism designed for precise leaflet grasp and coaptation. The launch marks a major advancement in India’s structural-heart therapy ecosystem and underscores Meril’s commitment to locally innovated cardiac devices.
In April 2025, Edwards Lifesciences announced that its SAPIEN M3 System had received the CE Mark approval, becoming the world’s first transfemoral transcatheter mitral valve replacement (TMVR) device indicated for symptomatic moderate-to-severe or severe mitral regurgitation in patients deemed unsuitable for surgery or TEER therapy. The system comprises a docking mechanism and the valve implant, delivered via a 29F steerable guide sheath through the femoral vein, establishing a new minimally invasive option in structural-heart care.
Key Players
Leading Companies of the Market
Abbott Laboratories
Edwards Lifesciences Corporation
Medtronic plc
Boston Scientific Corporation
Neochord, Inc.
4Tech Cardio
Tendyne Holdings, Inc.
Pi-Cardia, Inc.
Valtech Cardio
Cardiovalve Ltd.
In recent years, Abbott Laboratories has reinforced its leadership through iterative device enhancements and strategic partnerships with major U.S. cardiac centers, resulting in a 12% increase in device utilization in 2024 alone. Edwards Lifesciences initiated targeted acquisitions in 2023, expanding its product pipeline while securing exclusive patents that have strengthened competitive barriers. Medtronic’s investment in digitized patient monitoring technologies integrated with TMVR devices has led to improved procedural outcomes, enhancing its market share in hospitals worldwide.
Transcatheter Mitral Valve Repair And Replacement Market Future Outlook
The market is expected to witness strong expansion driven by the growing prevalence of mitral valve diseases, aging populations, and rising demand for minimally invasive cardiac care. Continuous product innovation will lead to next-generation systems featuring repositionable valves, enhanced biocompatible materials, and real-time AI-assisted navigation. Hybrid approaches that combine repair and replacement capabilities will likely emerge, offering tailored solutions for complex anatomies. The integration of digital imaging platforms, robotics, and remote monitoring technologies will further enhance precision and post-procedural outcomes. As reimbursement frameworks and clinical acceptance improve, the TMVR market will continue to move from niche to routine application in advanced cardiovascular care.
Transcatheter Mitral Valve Repair And Replacement Market Historical Analysis
The transcatheter mitral valve repair and replacement market has evolved significantly since the early 2000s, when percutaneous interventions first emerged as an alternative to high-risk open-heart surgery. Initial devices such as the MitraClip paved the way for minimally invasive cardiac therapies, focusing on patients with degenerative and functional mitral regurgitation. Early challenges in device durability, positioning, and patient selection limited adoption, but continuous advancements in imaging technologies, catheter design, and valve materials rapidly enhanced procedural outcomes. The integration of 3D echocardiography and fluoroscopic guidance further refined accuracy, while clinical trial success in high-risk and inoperable patient populations established TMVR as a mainstream treatment. Over time, collaborations between cardiovascular device manufacturers and healthcare providers expanded clinical experience, improving patient survival rates and procedural safety.
Sources
Primary Research Interviews:
Cardiologists
Cardiothoracic Surgeons
Interventional Radiologists
Biomedical Engineers
Hospital Procurement Specialists
Databases:
FDA Medical Device Database
ClinicalTrials.gov
PubMed Cardiology Studies
European Heart Journal Data Repository
EUDAMED Device Information
Magazines:
Medical Design & Outsourcing
Cardiology Today
MedTech Dive
DeviceTalks
Journals:
Journal of the American College of Cardiology (JACC)
Circulation
The Lancet Cardiology
Newspapers:
The New York Times (Health)
Financial Times (Science)
The Guardian (Health)
The Hindu (Science)
Associations:
American College of Cardiology (ACC)
European Society of Cardiology (ESC)
Society for Cardiovascular Angiography and Interventions (SCAI)
FDA CDRH
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients