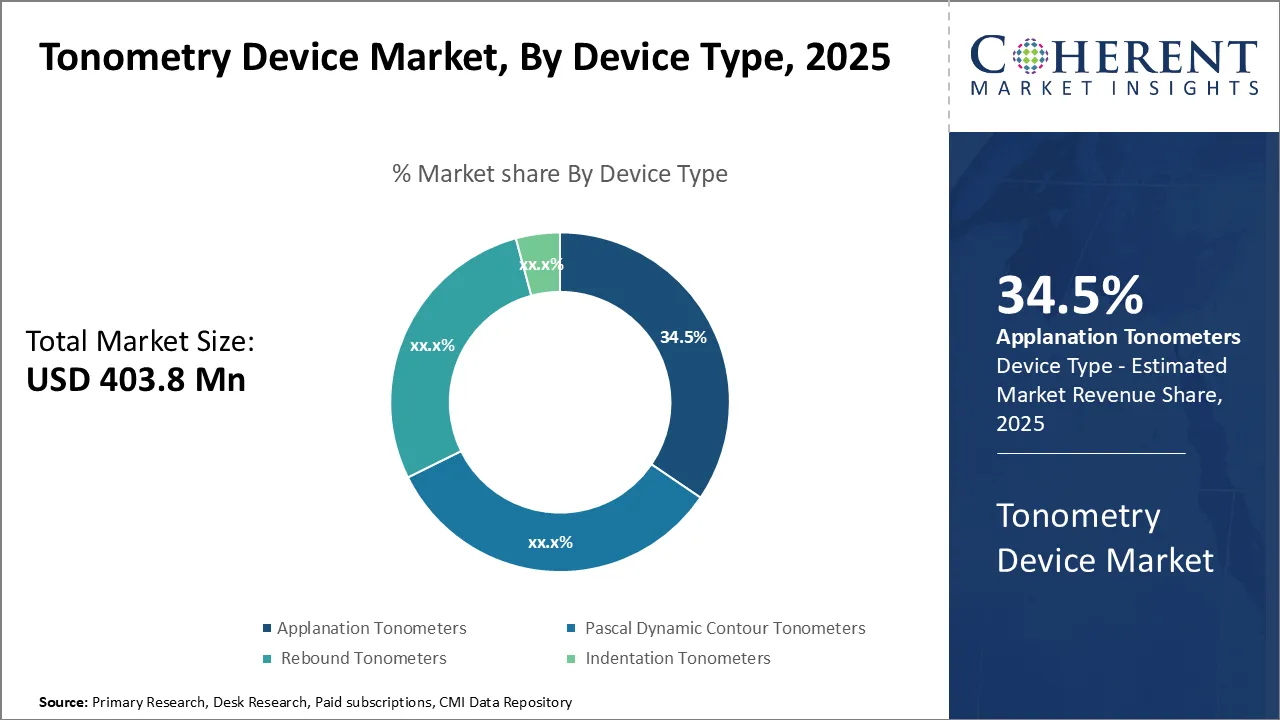
Tonometry devices market is estimated to be valued at USD 403.8 Mn in 2025 and is expected to reach USD 640.5 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 6.8% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
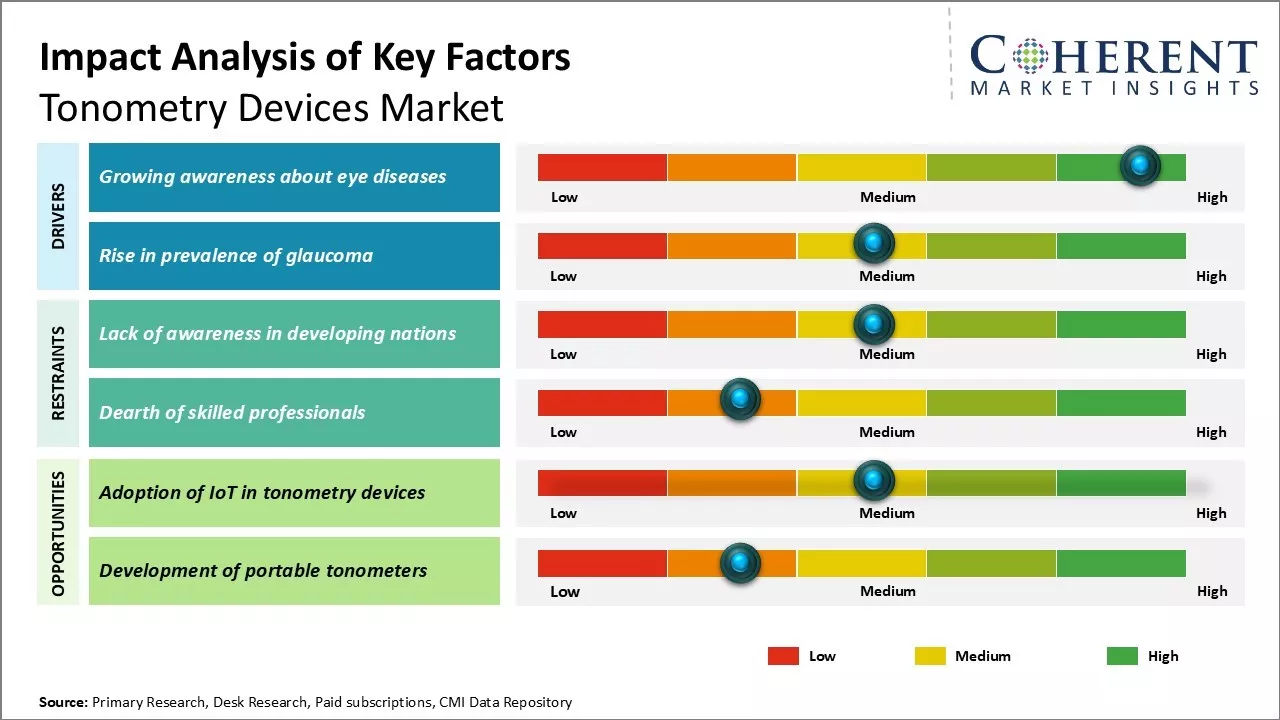
Rising prevalence of glaucoma and increasing demand for minimally invasive glaucoma surgeries (MIGS) can drive the tonometry devices market growth globally. Heightened healthcare expenditure and a rapidly growing geriatric population, who are at higher risk for glaucoma, further support the tonometry devices industry. However, lack of awareness about tonometry procedures in developing countries such as India can hamper the tonometry devices market growth. Increasing eye diseases prevalence can offer growth opportunities for tonometry devices market during the forecast period. The increasing prevalence of eye diseases presents substantial growth opportunities for tonometry devices during the forecast period, as more patients seek effective management options for their conditions.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
Insights, By Device Type- Applanation Tonometers Dominate Due to High Precision and Accuracy
In terms of device type, applanation tonometers segment is estimated to hold the highest market share of 34.5% in 2025, attributed to their precision and accuracy in measuring intraocular pressure (IOP). This technique flattens a specific area of the cornea, establishing it as the gold standard for clinical IOP measurement. Ophthalmologists favor applanation tonometers for routine glaucoma screening due to their reliable readings.
Insights, By Application- Glaucoma Diagnosis Dominates Due to Link between High IOP and Disease
In terms of application, glaucoma diagnosis segment is estimated to hold the highest market share of 30.2% in 2025, due to the established link between elevated intraocular pressure (IOP) and glaucoma. Accurate IOP measurement is vital for early detection, with ophthalmologists relying on tonometry exams to assess patients for optic nerve damage, making tonometry an essential tool in effective glaucoma management.
Insights, By Portability- Desktop Tonometers Dominate Due to Benefits of Stationary Units
In terms of portability, desktop tonometers segment is estimated to hold the highest market share of 56.7% in 2025, due to their reliability and consistency in measuring intraocular pressure (IOP). Widely used in hospitals and clinics, these stationary units minimize operator-dependent errors compared to handheld devices. Many models feature automated functions for self-adjustment, enhancing accuracy and ensuring standardized patient positioning for reproducible results.
Need a Different Region or Segment? Download Free Sample
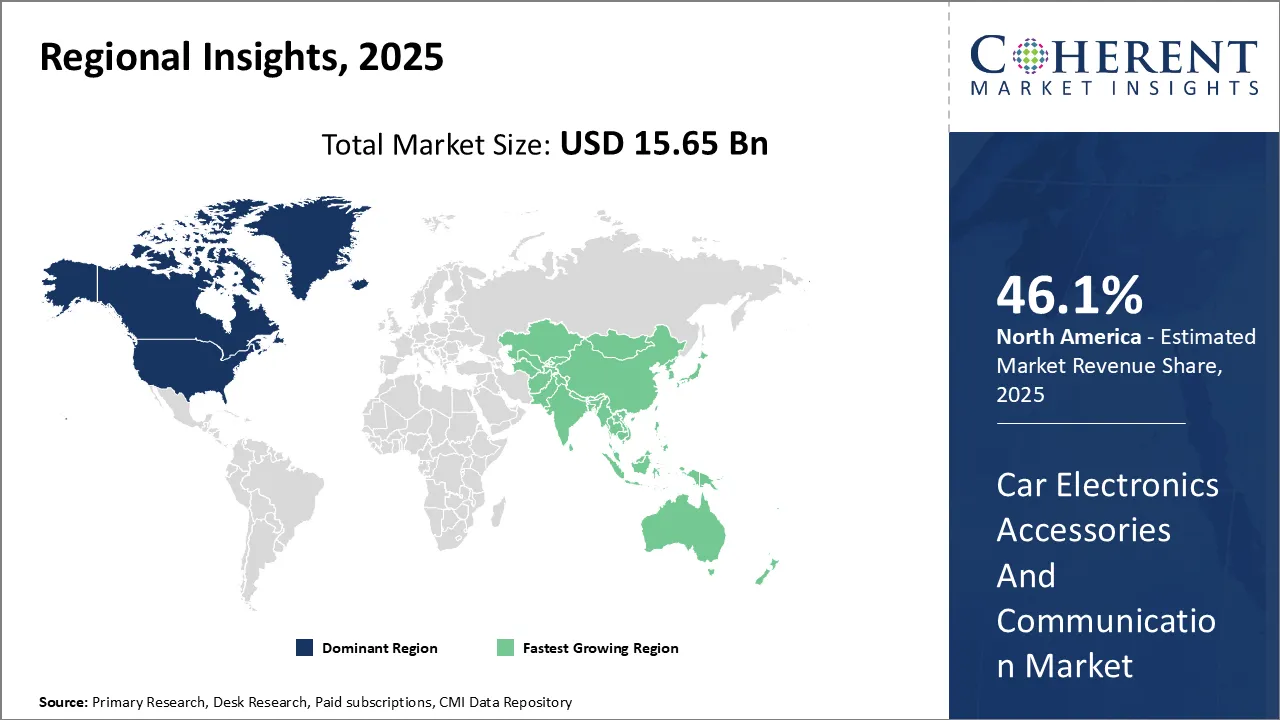
Dominating Region- North America
North America is expected to dominate the tonometry devices sector with an estimated market share of 37.9% in 2025, due to robust healthcare infrastructure and high adoption of advanced medical technologies. Presence of leading global players in the region has also boosted tonometry devices market growth.
Fastest-Growing Region- Europe
Europe exhibits the fastest growth with 28.8% market share in 2025, due to rising medical expenditures, increasing cases of ocular diseases, and growing awareness about eye care. The region's strong healthcare infrastructure and the presence of leading industry players further support this trend.
Tonometry Devices Market Outlook for Key Countries
High demand for technologically advanced tonometers in the U.S.
The U.S. tonometry devices industry is characterized by a strong demand for technologically advanced tonometers due to the rising prevalence of eye disorders, particularly glaucoma. Leading companies are focusing on product innovations to enhance device accuracy, ease of use, and patient comfort. This emphasis on advanced technology aims to improve diagnostic capabilities and streamline glaucoma management, positioning manufacturers for significant market growth.
China's market growth is supported by favorable government policies
China tonometry devices sector is experiencing growth driven by favorable government policies that promote local manufacturers. These initiatives encourage domestic companies to expand their product portfolios and enhance their global presence. Local firms are increasingly focusing on innovation and quality, positioning themselves to meet the rising demand for tonometry devices amid a growing prevalence of eye disorders like glaucoma.
India’s rising healthcare investments and increasing focus on ocular care infrastructure development
India continues to lead the South Asian market, driven by rising healthcare investments and a growing focus on developing ocular care infrastructure. Increased government initiatives and funding have facilitated the expansion of eye care facilities, enhancing access to tonometry services. This commitment to improving eye health is crucial for addressing the rising prevalence of glaucoma and other ocular disorders, ultimately improving patient outcomes across the region.
Japanese manufacturers are leveraging the country's highly skilled workforce to develop tonometers
Japanese manufacturers are leveraging the country’s highly skilled workforce to develop tonometers renowned for their precision and accuracy. Companies like Topcon Corporation and Takagi are leading the way, producing advanced devices that comply with international standards, such as the Goldmann applanation tonometer. Their commitment to innovation ensures that these tonometers meet the rigorous demands of modern ophthalmic care, enhancing diagnostic capabilities in glaucoma management.
Growing middle-class population and universal healthcare initiatives
Brazil tonometry devices sector is witnessing growth due to the expanding middle-class population and universal healthcare initiatives that enhance insurance coverage. Increased awareness about eye diseases, particularly glaucoma, is driving demand for tonometry devices. Additionally, government support for medical diagnostics is facilitating greater access to eye care services, further stimulating market expansion and improving patient outcomes across the country.
Get actionable strategies to beat competition: Download Free Sample
Top Strategies Followed by Tonometry Devices Market Players
Emerging Startups in the Tonometry Devices Market
Key Takeaways from Analyst
Tonometry Devices Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 403.8 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.8% | 2032 Value Projection: | USD 640.5 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
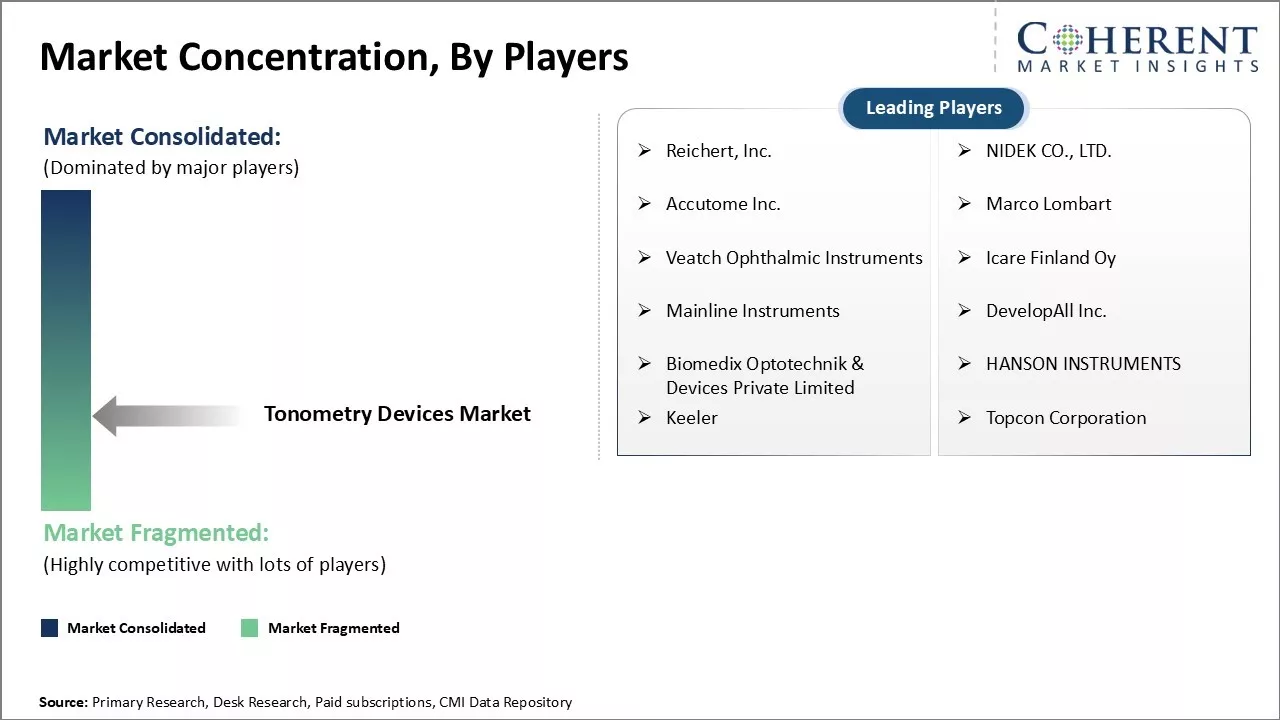
Reichert, Inc., NIDEK CO., LTD., Accutome Inc., Marco Lombart, Veatch Ophthalmic Instruments, Icare Finland Oy, Mainline Instruments, DevelopAll Inc., Biomedix Optotechnik & Devices Private Limited, HANSON INSTRUMENTS, Keeler, and Topcon Corporation |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Driver - Growing awareness about eye diseases
As eye care gains importance, awareness of diseases like glaucoma is increasing. Regular tonometry screenings are essential for early diagnosis, preventing vision loss. Non-profit organizations are conducting free eye check-up camps in urban and rural areas. Optometrists and ophthalmologists emphasize the importance of routine eye examinations for individuals of all ages, while social media helps disseminate information, encouraging regular eye tests as health consciousness rises.
Market Challenge- Lack of awareness in developing nations
The growth of the tonometry devices sector can be hindered by a lack of awareness about glaucoma and the importance of regular eye checkups, particularly in developing countries like India. Glaucoma is often referred to as a “silent” disease, presenting no symptoms until significant damage occurs, leading to late detection, higher treatment costs, and increased risk of vision loss.
Market Opportunity- Adoption of IoT in tonometry devices for market
Integration of Internet of Things (IoT) technology in tonometry devices presents a significant market opportunity. IoT-enabled tonometers can automatically upload eye examination results and patient details to cloud servers, facilitating remote monitoring of glaucoma patients. This data over time aids in analyzing disease progression and treatment effectiveness. Real-time sharing of readings allows for tele-consultations and predictive analytics, alerting physicians when readings exceed thresholds for early intervention.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients