Thyroid Disorder Therapy Market Size and Forecast – 2025 – 2032
The Global Thyroid Disorder Therapy Market size is estimated to be valued at USD 5.8 billion in 2025 and is expected to reach USD 10.4 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 8.6% from 2025 to 2032.
Global Thyroid Disorder Therapy Market Overview
Therapies for thyroid disorders include pharmaceutical and device-based products aimed at treating hypothyroidism, hyperthyroidism, thyroiditis, and thyroid cancer. Core product classes are hormone replacement therapies (levothyroxine tablets, liquid suspensions, pediatric dispersible formulations) for hypothyroidism; antithyroid drugs (methimazole, propylthiouracil) and beta-blockers for symptomatic control of hyperthyroidism; radioiodine (I-131) formulations and delivery systems for ablative therapy in Graves’ disease and thyroid cancer; and targeted therapies (tyrosine kinase inhibitors) for advanced thyroid malignancies.
Key Takeaways
The levothyroxine therapy segment continues to dominate, contributing over 42% to the market share due to its established efficacy and widespread prescription in hypothyroidism management.
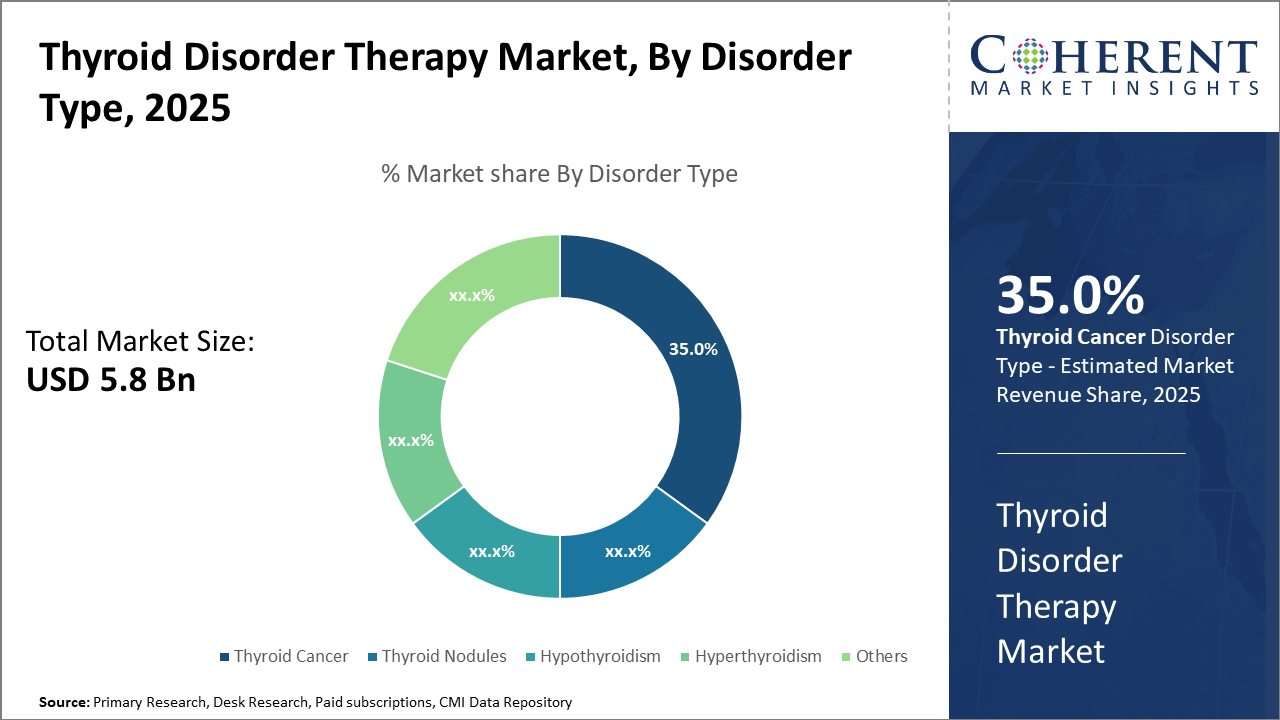
Thyroid cancer treatment represents a rapidly growing segment due to the rise in incidence and adoption of advanced targeted therapies, holding 35% of the disorder-specific market share.
Hospitals & Clinics are the largest distribution channel for thyroid disorder therapies globally, accounting for 48% of market revenue, driven by an increase in inpatient treatments and specialist consultations.
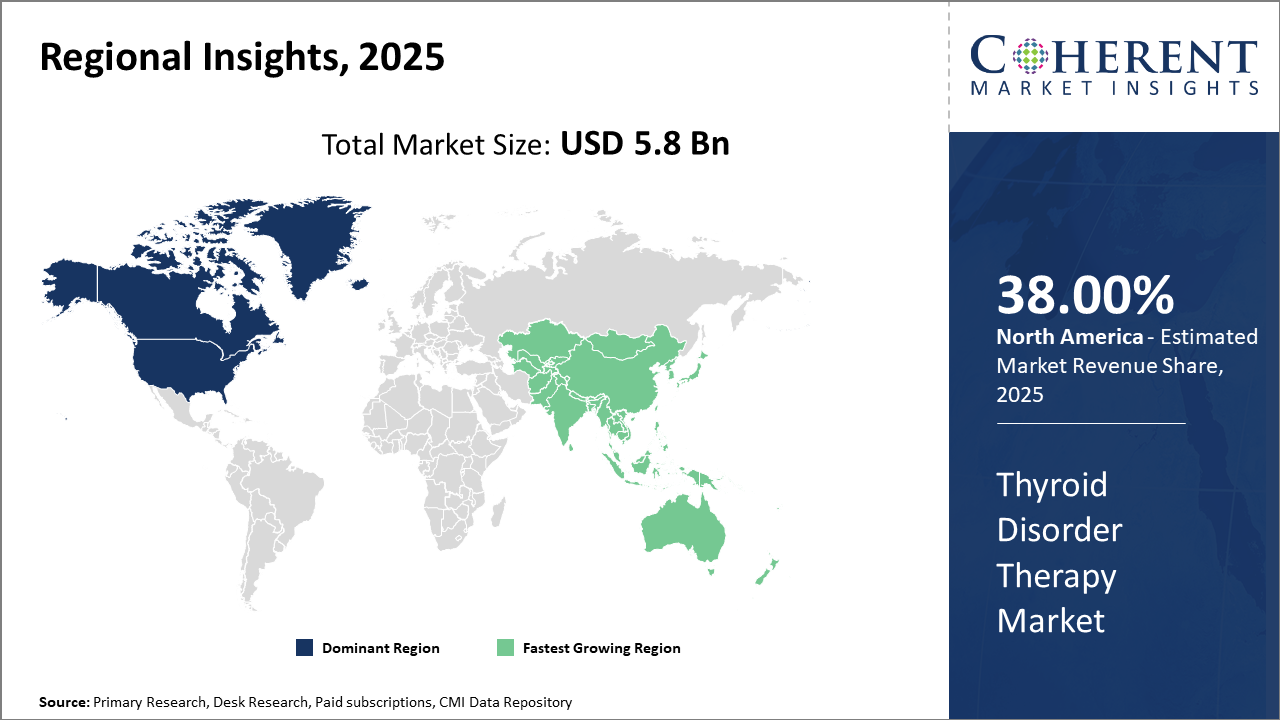
North America maintains market leadership with over 38% of the total thyroid disorder therapy market share, supported by advanced healthcare systems and proactive screening programs.
The Asia Pacific region is the fastest-growing market with a projected CAGR exceeding 9% driven by rising disease awareness, increasing healthcare expenditure, and expanding pharmaceutical manufacturing hubs in India and China.
Thyroid Disorder Therapy Market Segmentation Analysis
To learn more about this report, Download Free Sample
Thyroid Disorder Therapy Market Insights, By Therapy Type
Levothyroxine dominates the market share with 42%. Levothyroxine maintains dominance due to its status as the first-line treatment for hypothyroidism, bolstered by its proven efficacy and rising patient adherence in chronic therapy. The fastest growing subsegment is Radioactive Iodine Therapy, showing rapid adoption particularly in thyroid cancer treatments and hyperthyroidism management, benefitting from technological innovations that reduce radiation-related risks. Liothyronine serves as an adjunct therapy for refractory hypothyroid cases, while antithyroid drugs remain essential for hyperthyroidism control but face challenges due to side effects, which limit growth potential compared to other therapies.
Thyroid Disorder Therapy Market Insights, By Disorder Type
Thyroid Cancer commands 35% market share. Thyroid cancer’s dominance is attributed to rising incidence rates globally and advances in targeted molecular therapies improving survival outcomes. Hypothyroidism remains the largest patient population segment, but is fragmented due to differences in treatment regimens and disease severity. Among the fastest growing are thyroid nodules, driven by increased diagnostic imaging usage leading to early interventions. Hyperthyroidism treatments are steady yet face slow growth influenced by seasonal fluctuations and treatment side effects.
Thyroid Disorder Therapy Market Insights, By Distribution Channel
Hospitals & Clinics dominate the market share at 48%. This dominance reflects a preference for healthcare professional-administered treatments and the comprehensive monitoring required for thyroid therapies. The fastest growing channel is Online Pharmacies, facilitated by digital health adoption and increased patient preference for home delivery, especially post-COVID-19 disruptions in healthcare access. Retail Pharmacies offer accessibility and convenience, though growth is moderate due to rising online alternatives.
Thyroid Disorder Therapy Market Trends
The thyroid disorder therapy market is influenced by several impactful market trends. The increasing integration of digital health platforms for continuous hormone level monitoring enhances personalized treatment plans, especially in North America and Europe, during 2024 and 2025.
Additionally, progressive regulatory approvals for novel therapies, including tyrosine kinase inhibitors, have accelerated therapeutic advancements in thyroid cancer management globally.
Another trend is the growing patient preference for minimally invasive surgical techniques supported by recent clinical data from Japan and South Korea, reflecting reduced recovery times and complication rates.
These trends indicate a market ecosystem adapting to innovations and evolving patient-centric approaches.
Thyroid Disorder Therapy Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Thyroid Disorder Therapy Market Analysis and Trends
In North America, the dominance in the Thyroid Disorder Therapy market is attributed to an advanced healthcare infrastructure, well-established endocrine disorder screening protocols, and a strong pharmaceutical presence. The U.S. accounts for a substantial portion of the region’s market share of over 38%, supported by high healthcare expenditure and pioneering companies such as Pfizer and Mylan. Government-driven awareness programs and improved reimbursement policies have further spurred business growth and market revenue in this region.
Asia Pacific Thyroid Disorder Therapy Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth, with a CAGR surpassing 9%, fueled by rising thyroid disorder prevalence, increasing healthcare accessibility, and expanding pharmaceutical manufacturing hubs, particularly in India and China. Local companies like Lupin Limited and Sun Pharmaceutical have enhanced the market ecosystem through affordable drug formulations and strategic alliances, enabling rapid market penetration across diverse demographics.
Thyroid Disorder Therapy Market Outlook for Key Countries
USA Thyroid Disorder Therapy Market Analysis and Trends
The U.S. thyroid disorder therapy market is highly influential globally due to extensive R&D investments and high disease awareness levels. Recent data shows over 20 million people diagnosed with thyroid conditions, creating a strong demand for hormone replacement and targeted cancer therapies. Pfizer’s launch of improved levothyroxine formulations and AbbVie’s expansion of new drug pipelines have notably increased market revenue. The U.S. benefits from robust insurance coverage and an advanced clinical trials infrastructure, enabling rapid therapeutic adoption.
India Thyroid Disorder Therapy Market Analysis and Trends
India’s thyroid disorder therapy market is growing rapidly, driven by a large patient pool and expanding healthcare infrastructure. The government’s initiatives to address iodine deficiency disorders and increase endocrinologist availability have supported this growth. Local market players like Lupin Limited have capitalized on cost-effective therapies, gaining significant market share in urban and rural areas alike. The rising prevalence of thyroid cancers is also fostering demand for innovative treatments, making India a key regional growth market.
Analyst Opinion
The escalating prevalence of autoimmune thyroid diseases such as Hashimoto’s thyroiditis and Graves’ disease has been a fundamental driver for market growth. For instance, in 2024, the American Thyroid Association reported that approximately 20 million Americans suffer from some form of thyroid disease. This leads to increased prescription volumes of hormone replacement therapies that fundamentally impact the market share positively.
Rising demand for targeted therapies and personalized medicine in thyroid cancer treatment is reshaping the market landscape. In 2025, advancements in tyrosine kinase inhibitors (TKIs) usage and immunotherapies saw a 15% surge in adoption rates in oncology centers worldwide, particularly improving treatment outcomes for resistant and recurrent thyroid cancers, thereby influencing market revenue substantially.
The supply-side dynamics, including the expansion of pharmaceutical manufacturing capacities and increased import-export activity, have been central to fulfilling surging global demand. Between 2023 and 2025, active pharmaceutical ingredient (API) production for levothyroxine witnessed a capacity ramp-up by 12% in Asia, ensuring supply stability and competitive pricing, which benefits overall market growth strategies.
Evolving diagnostic and screening protocols have directly impacted market dynamics by earlier diagnosis and timely intervention. For example, nationwide screening programs in countries like South Korea and Germany expanded thyroid disorder detection rates by over 25% in 2024. This has expanded therapy adoption rates and enhanced market scope in these regions.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: |
USD 5.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.6% | 2032 Value Projection: |
USD 10.4 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Pfizer Inc., Mylan N.V., Bayer AG, Merck & Co., Inc., Allergan, Inc., Eli Lilly and Company, Dr. Reddy's Laboratories, Lupin Limited, Sun Pharmaceutical Industries Ltd., Teva Pharmaceutical Industries, Eisai Co., Ltd. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Thyroid Disorder Therapy Market Growth Factors
The increasing global incidence of thyroid disorders due to rising iodine deficiency and environmental factors continues to be a primary growth catalyst, with notable increases in hypothyroidism diagnoses reported in regions such as Southeast Asia in 2023. Secondly, technological advancements in drug delivery systems, including sustained-release formulations and injectable therapies, have made treatment more efficient, as evidenced by a 9% improvement in patient compliance rates reported in clinical studies in 2024. Thirdly, expanding healthcare infrastructure and favorable reimbursement policies, especially in North America and Europe, have expanded market accessibility, leading to a 12% increase in therapy adoption rates in hospital settings throughout 2025. Lastly, rising awareness campaigns by leading health organizations and government initiatives to promote thyroid health have positively impacted early detection and treatment, as shown by a 30% surge in thyroid screening tests in the U.K. in 2024.
Thyroid Disorder Therapy Market Development
In November 2025, Viridian Therapeutics announced the submission of its Biologics License Application (BLA) to the U.S. FDA for Veligrotug, a next-generation therapy for active Thyroid Eye Disease (TED). The filing marks a significant regulatory milestone following the FDA’s Breakthrough Therapy designation granted in May 2025, which recognized Veligrotug’s potential to offer faster and more durable improvements in proptosis and inflammatory symptoms compared to existing therapies. With its targeted IGF-1R inhibition and differentiated clinical profile, Veligrotug is positioned as a promising emerging treatment option for patients with active TED who require more effective, better-tolerated alternatives.
In May 2023, Indonesia launched the THYROID RAISE program, a nationwide initiative designed to expand access to diagnosis and treatment for hyperthyroidism and hypothyroidism by 2030. The program focuses on strengthening clinical capacity across primary and secondary care by training healthcare professionals, improving laboratory testing quality, and standardizing treatment protocols. By enhancing early detection and therapeutic management, THYROID RAISE aims to reduce the country’s large burden of undiagnosed thyroid disorders and support long-term endocrine health at a population level.
Key Players
Leading Companies of the Market
Pfizer Inc.
Mylan N.V.
Bayer AG
Merck & Co., Inc.
Allergan, Inc.
Eli Lilly and Company
Dr. Reddy's Laboratories
Lupin Limited
Sun Pharmaceutical Industries Ltd.
Teva Pharmaceutical Industries
Eisai Co., Ltd.
Competitive strategies among market companies focus on product innovation and geographic expansion. For example, in 2024, Pfizer launched a next-generation levothyroxine formulation that improved bioavailability, increasing its U.S. market share by 7% within 12 months. Similarly, Novartis has expanded its market presence in the Asia Pacific through strategic partnerships and localized clinical trials, boosting its market penetration in India and China. Several market players have also adopted cost leadership and differentiation strategies by investing in R&D to develop minimally invasive surgical tools and combination therapies, which have significantly enhanced patient outcomes and prescription adherence rates reported across European healthcare settings in 2025.
Thyroid Disorder Therapy Market Future Outlook
Future therapy developments will center on personalized dosing, novel targeted agents for refractory thyroid cancers, and improved delivery systems. Oral formulations that reduce absorption variability and pediatric-friendly dosing forms will expand. Advances in molecular oncology will bring more targeted tyrosine kinase inhibitors and immune-modulating approaches for advanced thyroid malignancies, while digital dose-management tools and pharmacogenomic-guided therapy may refine chronic hypothyroidism management.
Thyroid Disorder Therapy Market Historical Analysis
Therapies for thyroid disorders progressed from rudimentary iodine supplementation and surgical approaches to refined pharmacologic management. Levothyroxine replacement standardized hypothyroidism care in the mid-20th century, while antithyroid agents and radioiodine therapy provided effective control for hyperthyroidism and selected malignancies. Over recent decades, formulation innovations (liquid levothyroxine, soft-gel capsules) and tighter monitoring of TSH targets optimized therapeutic outcomes. Precision in managing subclinical disease and perioperative protocols also matured with improved diagnostics.
Sources
Primary Research Interviews:
Endocrinologists
Clinical pharmacologists
Thyroid surgeons
Pediatric endocrinologists
Databases:
PubMed Endocrinology literature
FDA drug approvals database
ClinicalTrials.gov (thyroid trials)
WHO Global Health Observatory
Magazines:
Endocrine Today
MedPage Today (Endocrinology)
The Endocrinologist
Clinical Endocrinology News
Journals:
The Journal of Clinical Endocrinology & Metabolism
Endocrine Reviews, Thyroid
Lancet Diabetes & Endocrinology
Newspapers:
The New York Times (Health)
Financial Times (Science)
The Guardian (Health), The Hindu (Health)
Associations:
American Thyroid Association (ATA)
Endocrine Society
European Thyroid Association (ETA)
World Health Organization (WHO)
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients