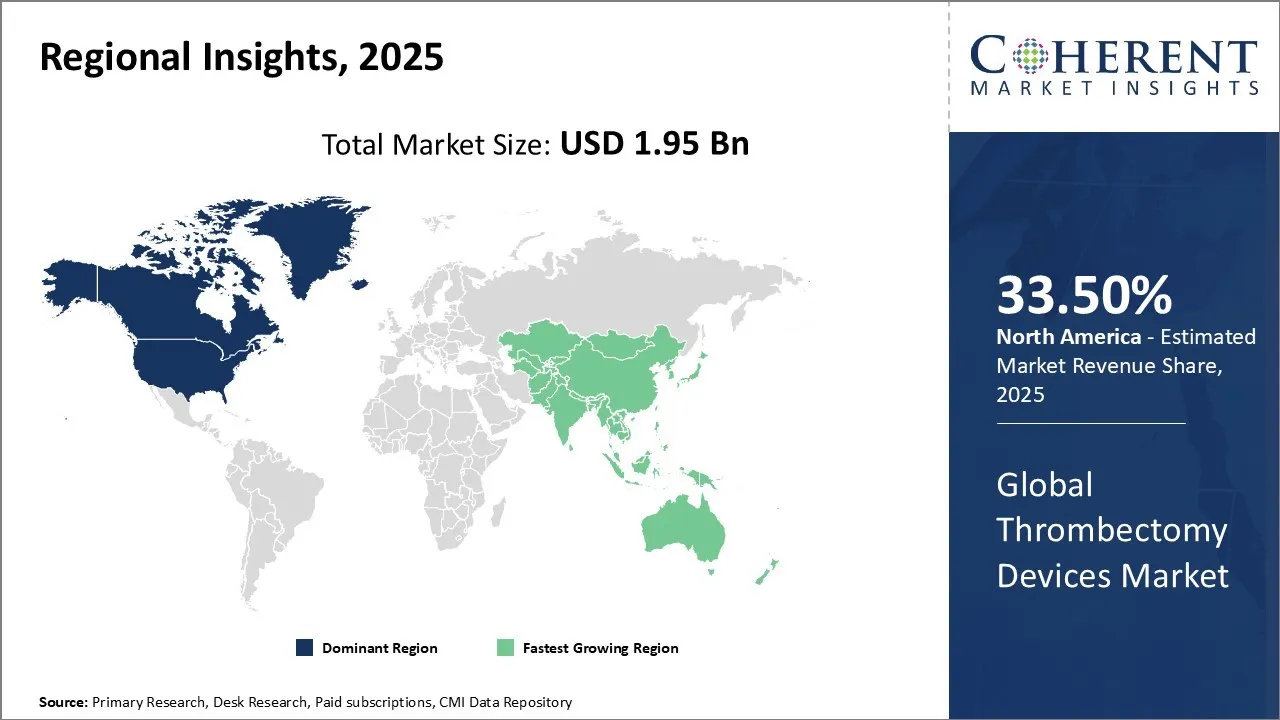
Thrombectomy Devices Market size is estimated to be valued at USD 1.95 Bn in 2025 and is expected to reach USD 3.24 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 7.5% from 2025 to 2032.
The global thrombectomy devices market demand is primarily driven by the increasing prevalence of cardiovascular diseases, including stroke and peripheral artery disease, which are often linked to blood clot formation. Furthermore, the aging population and a rise in lifestyle-related risk factors are contributing to the growing need for these devices. Thrombectomy can have a big impact in preventing and reducing long-term disability often caused by severe strokes. Some doctors call it a close to a miracle treatment. Thrombectomy is a life-changing treatment for stroke which patients can have within the first few hours of stroke. Thrombectomy is needed for someone who have blood clot in an artery or vein.
|
Current Event |
Description and its Impact |
|
Regulatory Shifts in Key Markets |
|
|
Epidemiological and Market Demand Shifts |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Reimbursement policies for thrombectomy devices vary by region but are increasingly supportive due to the clinical urgency and effectiveness of the procedure in treating conditions like acute ischemic stroke and deep vein thrombosis.
In the United States, the Centers for Medicare & Medicaid Services (CMS) covers mechanical thrombectomy under MS-DRG codes and has granted New Technology Add-on Payments (NTAP) for advanced systems like AI-enabled stroke detection tools. Physicians and facilities use CPT codes such as 37187, with average Medicare payments around $7,269 for facilities and $374 for physician services. In Japan, the Ministry of Health created a dedicated reimbursement category for devices like Inari Medical’s ClotTriever, providing premium coverage beyond standard catheter therapies.
In terms of product type, the mechanical thrombectomy devices segment is expected to hold a dominant position in the global thrombectomy devices market in 2025 with a share of 36.2% due to increasing product launches by key market players. Mechanical thrombectomy devices are in high demand due to the increasing prevalence of strokes and other cardiovascular diseases, coupled with advancements in device technology and proven clinical efficacy. These devices offer a minimally invasive way to remove blood clots, potentially minimizing long-term disability and improving patient outcomes. The effectiveness of mechanical thrombectomy on patient has been relatively improved over time and their MRD index decreased significantly.
In March 2025, Stryker, through its Inari Medical division, launched the ARTiX Thrombectomy System, a dual-mode device combining aspiration and mechanical techniques for treating arterial thromboembolism. Designed for peripheral arterial blockages, the low-profile, over‑the‑wire 8 Fr sheath includes a covered nitinol mesh funnel to prevent clot migration.
In terms of application, the deep vein thrombosis (DVT) segment is expected to dominate the market over the forecast period due to increasing prevalence of deep vein thrombosis. Deep vein thrombosis (DVT) is a serious condition because the blood clots that form in deep veins can break loose and travel to the lungs, causing a pulmonary embolism (PE), which can be life-threatening. Using thrombectomy device is used to treat deep vein thrombosis (DVT) which is particularly effective in cases of iliofemoral DVT or when patients are at high risk for complications like pulmonary embolism or post-thrombotic syndrome (PTS). Thrombectomy offers significant advantages, including rapid symptom relief, preservation of vein function, and reduced risk of bleeding compared to systemic thrombolysis.
In December 2024, Inari Medical announced that Japan’s Ministry of Health, Labor and Welfare approved national reimbursement for its ClotTriever Thrombectomy System for deep vein thrombosis (DVT) treatment. The approval establishes a new functional category with premium reimbursement, acknowledging the device’s unique wall-to-wall thrombus removal capabilities and strong clinical safety and efficacy data.
In terms of end user, the hospitals segment is expected to dominate the market over the forecast period due to increasing number of hospitals for thrombectomy process. Thrombectomy devices are increasingly in demand in hospitals due to their ability to deliver rapid, effective, and minimally invasive treatment for life-threatening conditions like acute ischemic stroke, deep vein thrombosis (DVT), and pulmonary embolism (PE). These conditions require immediate restoration of blood flow to prevent irreversible damage or death, making thrombectomy a critical intervention tool in emergency and intensive care settings. Hospitals are also seeing benefits in terms of cost efficiency and patient outcomes. Shorter ICU stays, faster mobilization, and reduced rates of post-thrombotic syndrome or long-term disability are driving hospitals to integrate thrombectomy into their interventional radiology, cardiology, and neurovascular protocols. This is further accelerating the thrombectomy market share.
To learn more about this report, Download Free Sample
North America is estimated to hold a dominant position in the global thrombectomy devices market over the forecast period. North America is estimated to hold 33.50% of the market share in 2025. North America thrombectomy devices market is expected to witness significant growth in the near future due to high prevalence of pulmonary embolism in the region. Increasing prevalence of pulmonary embolism among the patients is expected to drive the North America thrombectomy devices market growth over the forecast period. According to American Lung Association, in the United States, pulmonary embolism (PE), which is a blockage of an artery in the lungs, is a serious and potentially fatal condition. It is estimated to affect approximately 900,000 people annually and is the third leading cause of cardiovascular disease-related death. According to Canada’s Drug Agency, the incidence rate of pulmonary embolism in Canada is more than 1 in 1,000 (0.1%), and less than 1 in 100 (1%). This is further propelling the thrombectomy market share.
The demand for thrombectomy device market is rising due to the increasing prevalence of cardiovascular and cerebrovascular diseases, improvements in clinical guidelines, and growing investments in healthcare infrastructure aimed at early intervention and improved outcomes. In European countries like Germany, France, and the UK have seen significant adoption of mechanical thrombectomy as a standard of care for large vessel occlusions. This follows updated European Stroke Organisation (ESO) guidelines that recommend thrombectomy as the first-line treatment within specific time windows. Hospitals across Europe have responded by equipping stroke centers with devices like the Solitaire™, Trevo®, and EmboTrap®, enabling faster, more effective clot retrieval.
Furthermore, Europe’s strong emphasis on minimally invasive procedures and value-based care supports the uptake of thrombectomy devices that reduce hospital stays, lower complications, and speed up recovery. Increased funding for stroke-ready hospitals, national stroke plans (e.g., in the UK and Sweden), and cross-border telemedicine networks also fuel adoption by ensuring timely diagnosis and treatment.
The United States is witnessing a significant surge in demand for thrombectomy devices, largely due to the high prevalence of stroke and venous thromboembolism (VTE), which remain among the leading causes of mortality and long-term disability. Each year, nearly 800,000 Americans suffer a stroke, with a substantial portion attributed to large vessel occlusions, which require rapid intervention. Simultaneously, cases of deep vein thrombosis and pulmonary embolism are rising due to factors such as aging, obesity, and sedentary lifestyles.
In January 2025, Argon Medical Devices initiated a clinical trial by enrolling the first patient to evaluate a new catheter-based device designed to treat pulmonary embolism (PE), a potentially fatal blockage of the lungs’ blood vessels. This U.S.-based study aims to assess the device’s safety and efficacy in mechanically extracting blood clots. Harnessing minimally invasive technology, the catheter promises a faster, potentially safer alternative to traditional treatments such as systemic thrombolysis or surgical intervention. Results from this trial could reshape standards of care for PE.
Germany has emerged as one of the leading markets for thrombectomy devices in Europe, largely due to its significant stroke burden and strong governmental support for advanced interventional treatments. Stroke remains a major public health concern in Germany, with thousands of cases annually requiring immediate and effective intervention. In response, the German healthcare system has prioritized the expansion of mechanical thrombectomy as a frontline treatment for acute ischemic stroke. Currently, the country has more than 300 stroke-ready hospitals equipped with neurointerventional capabilities, ensuring widespread access to life-saving procedures, which is further proliferating the thrpmbectomy market revenue.
In India, the demand for thrombectomy devices is steadily increasing, driven by the rising incidence of stroke and deep vein thrombosis (DVT) across urban and semi-urban populations. The incidence of stroke in India ranges from 119 to 145 per 100,000 population annually, with a higher prevalence observed in urban areas compared to rural regions. At the same time, the country has seen a rapid growth in neuro-interventional and vascular centers, particularly within the private healthcare sector, enabling more hospitals to offer mechanical thrombectomy procedures. For instance, in June 2025, Pioneering Neurointerventional Excellence at Jehangir Hospital in Pune has introduced advanced Neurointerventional Service, equipped with would-class infrastructure, expert clinicians and cutting-edge technology.
In May 2025, the Government of India, via the Technology Development Board (Department of Science & Technology), announced funding support for S3V Vascular Technologies’ development of the nation’s first mechanical thrombectomy kit for acute ischemic stroke. This initiative offers a more affordable, locally manufactured alternative to costly imports, aligning with Ayushman Bharat reimbursement schemes. With CE and USFDA approvals underway, S3V aims to enhance stroke care access across India and global markets.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.95 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.5% | 2032 Value Projection: | USD 3.24 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Boston Scientific Corporation, Medtronic, Merit Medical System Inc., Stryker Corporation, Terumo Corporation, Teleflex Incorporated, Vetex Medical Ltd., Edwards Lifesciences Corporation, Penumbra Inc., Control Medical Technology, LLC., Rapid Medical, Abbott and Surmodics, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Increasing product approval by regulatory authorities such as China’s National Medical Product Administration is expected to drive the global thrombectomy devices market growth over the forecast period. For instance, on August 31, 2023, Rapid Medical, a Israel-based developer of neurovascular devices, announced that its Tigertriever revascularization device had received approval from China’s National Medical Product Administration. Tigertriever provides patient-specific solutions for removing blood clots from the brain in the treatment of ischemic stroke.
Moreover, increasing product approvals by regulatory authority such as U.S. Food and Drug Administration are also expected to boost the global thrombectomy devices market in North America region. For instance, on June 14, 2023, Surmodics Inc., announced that the U.S. Food and Drug Administration had received approval for Pounce LP Thrombectomy System that is intended for the non-surgical removal of thrombi and emboli from the peripheral arterial vasculature in vessels 3.5 mm to 6 mm in diameter. The Pounce LP Thrombectomy System, a new addition to the Pounce platform, is indicated for use in vessels, ranging from 2 mm to 4 mm in diameter, sizes typical of vessels found below the knee.
The thrombectomy devices market forecast is poised for strong growth, driven by next-gen innovations and expanding applications. Devices are becoming smaller, safer, and more precise, with integration of AI and real-time imaging for better outcomes. Beyond stroke, usage is increasing in pulmonary embolism, DVT, and PAD. Emerging markets like India and China are expanding access as stroke care infrastructure improves. Recent FDA recalls are also prompting safer designs and stricter quality standards. Future developments may include hybrid devices combining aspiration and clot disruption. Overall, thrombectomy is evolving into a core tool for treating multiple acute vascular conditions.
The thrombectomy devices market value is approaching a pivotal inflection point, driven by clinical efficacy, evolving reimbursement frameworks, and the unmet urgency in acute ischemic stroke (AIS) management. What was once a technology reserved for a narrow cohort of neurovascular interventions is now broadening in scope both anatomically and geographically with significant implications for clinical adoption and device innovation.
One cannot overstate the paradigm-shifting role of mechanical thrombectomy in stroke treatment. Recent randomized trials, including the RESCUE-Japan LIMIT and SELECT2 studies, have not only validated thrombectomy for large core infarcts but are pushing the boundaries of patient inclusion criteria. The implication is straightforward: procedural volumes will expand, and so will demand for devices capable of crossing tortuous anatomy and fragmenting complex thrombi without inducing vessel trauma.
Yet, the market is constrained by product complexity and operator variability. It is here that companies like Penumbra and Stryker continue to hold an advantage—not because they lead in R&D alone, but because they control training ecosystems through procedural simulation, which standardizes outcomes and entrenches their devices in the neuro-interventional toolkit. The aspiration-catheter-based Indigo and JET systems are rapidly becoming gold standards for peripheral and pulmonary embolism interventions, respectively. This positions these companies not just as product vendors, but as clinical partners, a position that newcomers find hard to replicate.
In summary, the thrombectomy devices market will no longer be defined by who manufactures the best catheter. It will be led by those who can lower the learning curve, demonstrate cost-offset through improved long-term neurological outcomes, and link devices with digital platforms that enable precision stroke care. Players not adapting to this clinical and technological convergence will struggle to defend share in a market becoming increasingly outcomes-driven.
*Definition: Thrombectomy involves removing a clot from a blood vessel, most commonly from the brain, heart, or lungs. Stroke and myocardial infarction are two of the leading causes of death worldwide, and are increasingly treated through techniques that include intravascular thrombolytics, mechanical thrombectomy, or a combination of both.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients