Tapentadol - Become Pain Free!
Tapentadol is an opioid analgesic, indicated for severe pain management, on a daily or around the clock need for treatment and long-term opioid treatment, for which alternative treatment options are inadequate. Tapentadol is also indicated for neuropathic pain associated with diabetic peripheral neuropathy. It is widely used for management of acute, chronic, and cancer related pain among patients and is available legally on prescription. Nucynta ER is the first and only FDA-approved long-acting opioid analgesic designed to control both, nociceptive pain and neuropathic pain associated with diabetic peripheral neuropathy. Nucynta is available as Palexia outside the U.S. territory. Nucynta ER is contraindicated for patients suffering from significant respiratory depression, known or suspected gastrointestinal obstruction, acute or severe bronchial asthma or hypercarbia, including paralytic ileus, concurrent use of monoamine oxidase inhibitors (MAOIs), and Hypersensitivity (e.g. anaphylaxis, angioedema) to tapentadol or to any other ingredients of the product or use of MAOIs within a period of 14 days.
Rising incidence of cancer and diabetic neuropathy in the Middle East is expected to fuel growth of the Middle East tapentadol market
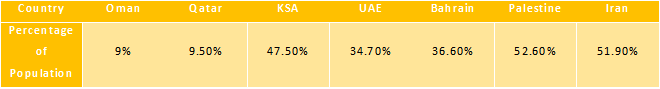
According to data published in World Journal of Diabetes in 2016, the Middle Eastern and North African regions are estimated to have the second-highest rate of diabetes in the world, in turn, increasing prevalence of neuropathic pain. In Arab World, the prevalence of diabetes is projected to increase to 96.2% by 2035, as estimated by World Journal of Diabetes in 2016. Furthermore, the World Health Organization (WHO) estimated that in the Eastern Mediterranean region, including Middle East, recorded 555,318 new cases of cancer in 2012, which is projected to rise to 961,098 by 2030. Thus determining that the Eastern Mediterranean region is expected to have the highest relative increase of cancer cases among all regions in the world. According to Journal of Taibah University Medical Sciences, 2016, the number of people suffering from diabetic neuropathic pain in countries of the Middle East were as follows:
To learn more about this report, Download Free Sample
The Middle East tapentadol market was valued at US$ 0.82 million in 2016 and is expected to witness a robust CAGR of 6.1% over the forecast period (2017-2025).
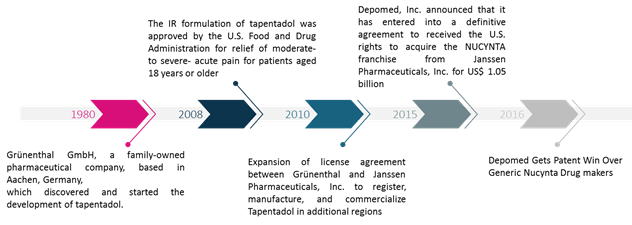
Figure 1. Tapentadol related important events since discovery
To learn more about this report, Download Free Sample
Absence of gold standard opioid therapy for neuropathic pain other than tapentadol is expected to fuel the Middle East tapentadol market growth
Neuropathic pain is caused by a dysfunction of or lesion to the central or peripheral nervous system and it is chronic in nature with a complex pathophysiology. NUCYNTA ER (tapentadol) is used in the treatment of neuropathic pain, if the pain is severe enough to require tapentadol daily, whereas it is also used in long-term opioid treatment for which alternative treatment options are inadequate. There is low quality evidence that the oral tramadol has any important beneficial effect on pain for people suffering from severe or moderate neuropathic pain, whereas the main indication of tapentadol is to treat the diabetic neuropathic pain. This proves that other opioids such as fentanyl, oxycodone, tramadol, and oxymorphone are not as effective as tapentadol in the treatment of neuropathic pain.
The U.S. Food & Drug Administration (FDA) tapentadol approval timeline

To learn more about this report, Download Free Sample
Major players operating in the tapentadol market include Depomed, Inc., Janssen Pharmaceutical, Inc., Grünenthal, and Allergan Plc.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients