The systemic scleroderma treatment market is estimated to be valued at USD 1.80 Bn in 2025 and is expected to reach USD 2.55 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 5.1% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
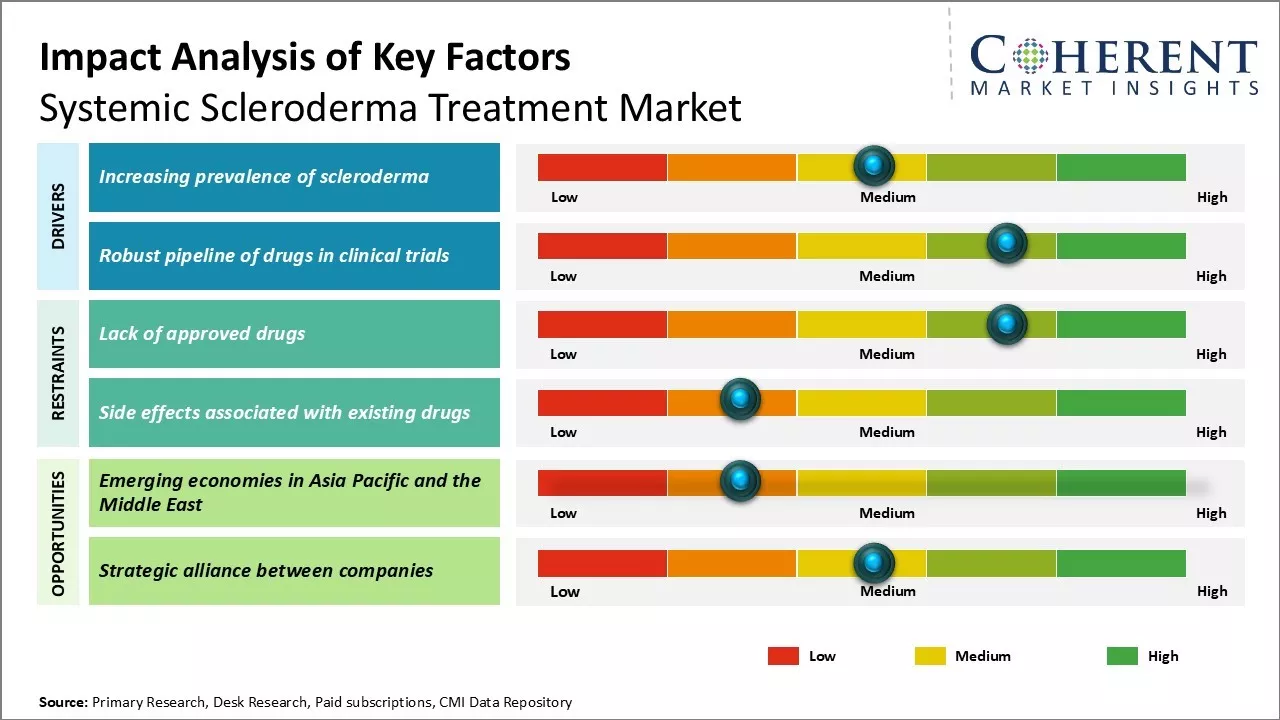
The systemic scleroderma treatment industry is experiencing significant growth driven by the rising incidence of the disease and increased government funding for research. As awareness of systemic scleroderma expands, more patients are seeking treatment options, leading to a higher demand for effective therapies. Government initiatives and funding are facilitating research and development of new treatments, further propelling the market expansion. However, challenges such as the lack of curative therapies and high costs associated with advanced treatments may restrain the market growth.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
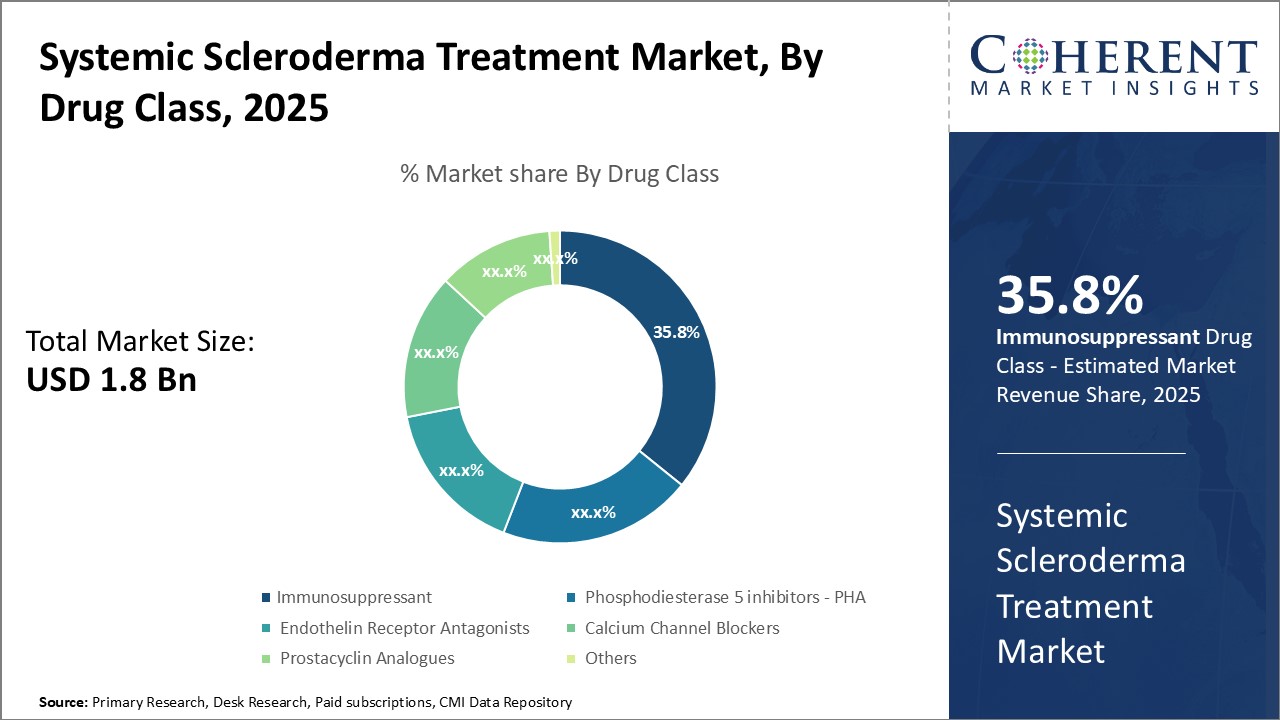
Insights By Drug Class - Innovation drives immunosuppressant segment’s dominance
In terms of drug class, immunosuppressant segment is expected to contribute the highest market share of 35.8% in 2025, due to ongoing innovations in drug development. Immunosuppressants are crucial for modulating the immune system. Substantial investments by leading pharmaceutical companies in research and development have led to novel therapies, including biosimilars and specialty drugs like mycophenolate mofetil and cyclophosphamide, which target B and T cells effectively.
Insights By Type - Awareness drives limited scleroderma segment’s dominance
In terms of type, limited scleroderma segment is expected to contribute the highest market share of 45.7% in 2025. Increasing disease awareness and the rising prevalence of systemic scleroderma are driving this segment growth. Moreover, earlier diagnosis of this autoimmune disease contributes to this segment growth.
Insights By Route of Administration - Convenience leads oral segment’s dominance
In terms of route of administration, oral segment is expected to contribute the highest market share of 69.7% in 2025, due to convenience in administration. Systemic scleroderma poses challenges with frequent medical interventions leading to poor medication adherence. However, oral drugs provide hassle-free self-administration at home settings. This has boosted preference over injectable drugs requiring hospital visits.
Need a Different Region or Segment? Download Free Sample
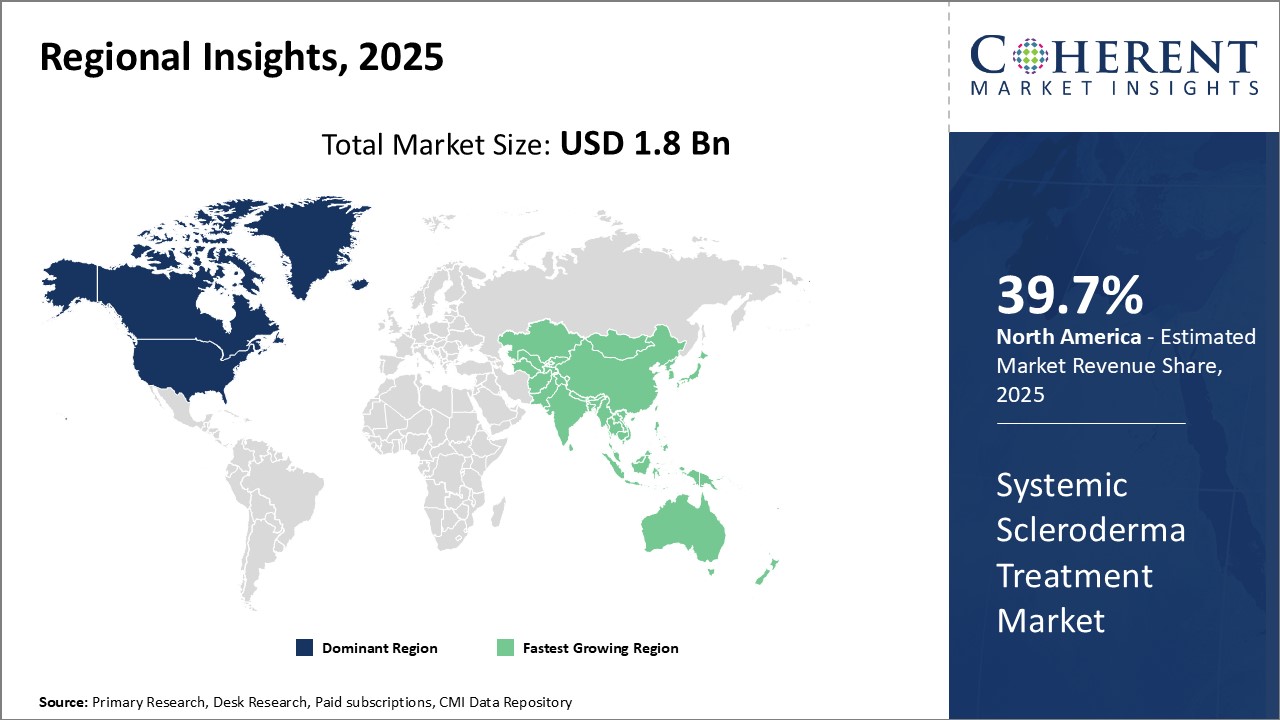
Dominating Region: North America
North America is expected to hold the highest market share of 39.7% in 2025. This can be attributed to a strong presence of leading pharmaceutical companies and growing research and development activities in the region.
Fastest-Growing Region: Asia Pacific
The Asia Pacific region exhibits the fastest growth with 24.1% market share in 2025 and is expected to emerge as a lucrative market in coming years. This can be attributed to rising healthcare expenditure, growing medical tourism industry, and increasing focus of global players to tap into developing healthcare markets.
Systemic Scleroderma Treatment Market Outlook for Key Countries
Increasing product launches/approvals in the U.S.
U.S. systemic scleroderma treatment industry is largely fueled by new product approvals and launches. Companies are significantly expanded treatment options, enhancing patient access to innovative therapies. This dynamic environment fosters competition and encourages ongoing advancements in treatment methodologies, ultimately benefiting patients and healthcare providers alike.
High disease prevalence in Japan
Japan plays a crucial role in the systemic scleroderma treatment industry, driven by its high disease prevalence and a strong emphasis on developing orphan drugs. The country's optimized regulatory pathways facilitate quicker access to innovative therapies, enhancing treatment options for patients. This focus on rare diseases fosters significant investment in research and development, further propelling market growth.
China’s high patient population and high prevalence of the disease
China remains a leading market for systemic scleroderma treatment, largely due to its significant patient population and high prevalence of the disease. This demographic presents key opportunities for stakeholders to enhance their presence in the region. Ongoing research and development efforts focused on innovative therapies further strengthen the market's potential, attracting investment and fostering collaboration among healthcare providers.
India’s favorable regulatory guidelines
The India systemic scleroderma treatment sector is rapidly expanding, driven by favorable regulatory guidelines and the increasing medical needs of a large patient population. The government's supportive policies facilitate quicker access to innovative therapies, while the growing awareness of systemic scleroderma prompts more patients to seek effective treatment options, further boosting market growth.
Get actionable strategies to beat competition: Download Free Sample
Top Strategies Followed by Systemic Scleroderma Treatment Market Players:
Emerging Startups in the Systemic Scleroderma Treatment Market:
Key Takeaways from Analyst
Systemic Scleroderma Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.80 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.1% | 2032 Value Projection: | USD 2.55 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
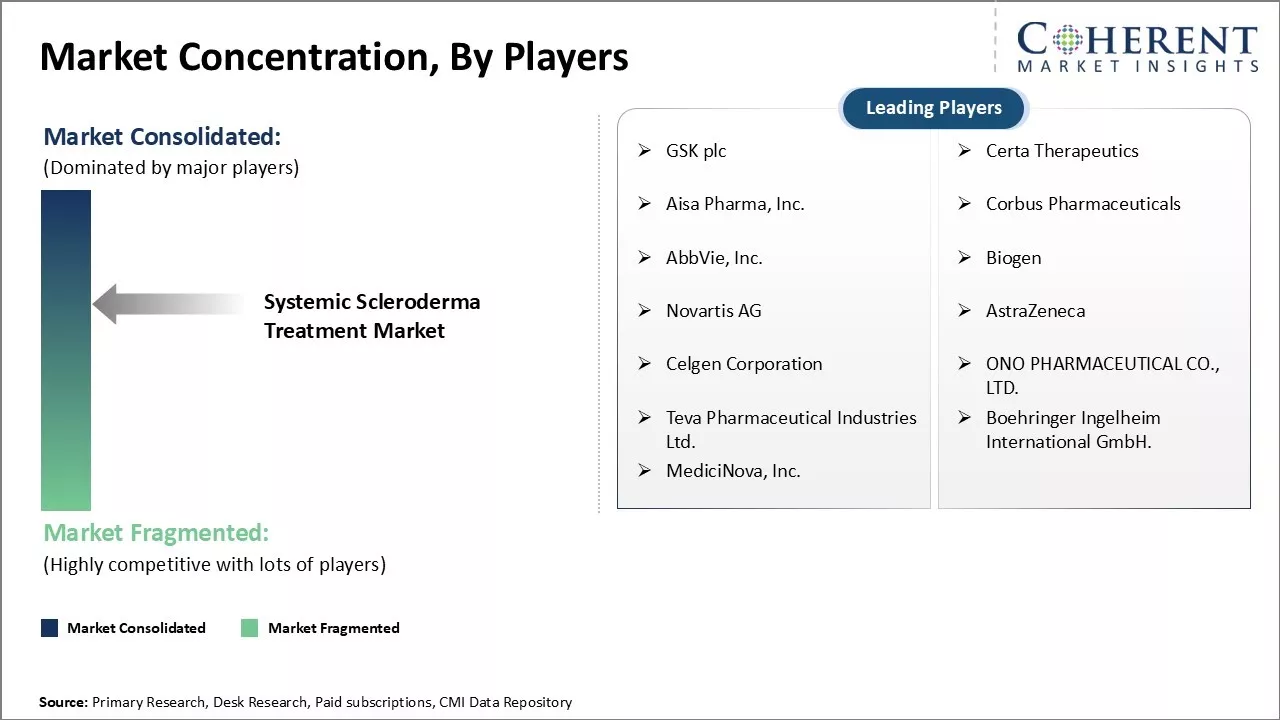
| Companies covered: |
GSK plc, Certa Therapeutics, Aisa Pharma, Inc., Corbus Pharmaceuticals, AbbVie, Inc., Biogen, Novartis AG, AstraZeneca, Celgen Corporation, ONO PHARMACEUTICAL CO., LTD., Teva Pharmaceutical Industries Ltd., Boehringer Ingelheim International GmbH., and MediciNova, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Driver - Increasing prevalence of scleroderma
The prevalence of systemic scleroderma has been steadily rising globally, driven by genetic and environmental factors. This life-threatening connective tissue disorder affects the skin and internal organs, leading to significant health complications. The increasing patient population emphasizes the urgent need for improved treatment options to manage this non-curable condition effectively.
Market Challenge - Lack of approved drugs
The systemic scleroderma treatment market faces significant challenges due to the absence of approved drugs specifically targeting the disease. Current therapies mainly manage symptoms, while the complex pathogenesis complicates drug development. High attrition rates, lengthy development cycles, and difficulties in patient enrollment hinder clinical research, limiting treatment options and market growth. Substantial investments in R&D are essential to create effective therapies that can modify the disease's progression.
Market Opportunity - Emerging economies in Asia Pacific and Middle East
The systemic scleroderma treatment market is poised for growth in emerging economies within the Asia Pacific and the Middle East regions. Rapidly developing healthcare infrastructures, increasing medical expenditures, and heightened awareness of autoimmune disorders create lucrative opportunities. Local pharmaceutical companies are actively licensing innovative drug candidates, while favorable regulations and lower development costs attract international firms for clinical trials, enhancing global drug development contributions. This evolving landscape promises long-term benefits for the systemic scleroderma treatment industry.
What does Growth in the Systemic Scleroderma Treatment Industry Mean for Different Stakeholders?
The systemic scleroderma treatment industry has multiple players with varied designations and offers multiple opportunities based on their scope of operations.
|
Key Pharmaceutical Stakeholder |
Opportunities Due to Systemic Scleroderma Treatment Industry Growth |
|
Retail Pharmacies |
Expansion of product offerings to include new drugs and personalized medicine solutions, enhancing customer care and market reach. |
|
Chemical Suppliers |
Growth in demand for specialty chemicals used in drug synthesis, including organic intermediates, catalysts, and reagents. |
|
Pharmaceutical Companies |
Expansion of product pipelines with new drug discoveries, biologics, and biosimilars, capitalizing on growing global healthcare needs. |
|
Contract Research Organizations (CROs) |
Increased outsourcing of clinical trials and drug development, offering opportunities for growth and long-term partnerships. |
|
Contract Manufacturing Organizations (CMOs) |
Growing demand for scalable manufacturing solutions, including biologics production and complex drug formulations. |
|
Diagnostic Equipment Manufacturers |
Expanded markets for diagnostic tools and devices that support personalized medicine and early disease detection. |
|
Healthcare Providers |
New treatment options and innovative therapies, improving patient care and expanding healthcare services. |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients