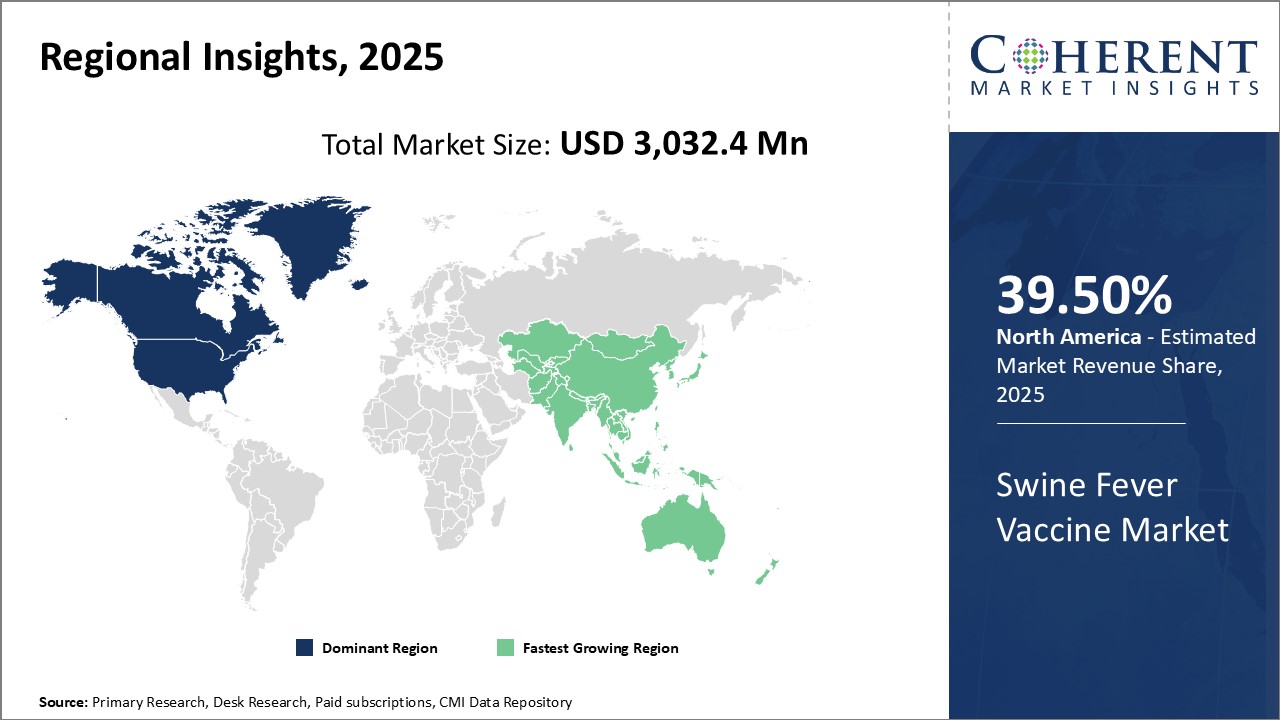
The global swine fever vaccine market is estimated to be valued at USD 3,032.4 Mn in 2025 and is expected to exhibit a CAGR of 6.1% during the forecast period (2025-2032). African swine fever (ASF) and classical swine fever (CSF) are highly contagious viral diseases of pigs. They are clinically identical and cannot be definitively distinguished from one other in the field. A diagnosis must be verified by laboratory tests. These diseases may seem to be identical; however, they are caused by totally unrelated viruses. CSF, often called as 'hog cholera', is caused by a virus from the pestivirus genus of the family Flaviviridae and is closely related to the virus that causes bovine viral diarrhea (mucosal disease) in cattle and border disease (hairy shaker disease) in sheep. The virus that causes ASF is unrelated to the virus that causes classical swine fever and has a more complex genetic makeup. Pigs are the only animals affected by both ASF and CSF; humans and other animals are not affected by these diseases.
Figure 1. Global Swine Fever Vaccine Market Value (USD MN), by Region, 2025
To learn more about this report, Download Free Sample
Increasing outbreaks of swine fever are expected to drive the swine fever vaccine market growth.
The increasing outbreaks of swine fever is expected to drive the market growth over the forecast period. For instance, as of May 9, 2025, 259 pigs have died in Meghalaya’s Ri-Bhoi district in India due to African Swine Fever (ASF), according to a senior official of the Veterinary and Animal Husbandry Department. The first outbreak was reported by the Veterinary department on April 13, 2025 following which instructions were issued under the Prevention and Control of Infectious and Contagious Diseases in Animals Act, 2009, banning movement of pigs, slaughter, and supplies at Umshorshor village, Meghalaya, India, and within 10 km of its vicinity.
Swine Fever Vaccine Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3,032.4 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.1% | 2032 Value Projection: | USD 4,589.8 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Merck & Co., Inc. (Merck Animal Health), Ceva, Zoetis Services LLC, Boehringer Ingelheim International GmbH, Indian Immunologicals Ltd., Bioveta, a.s., Komipharm, and EC21 Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
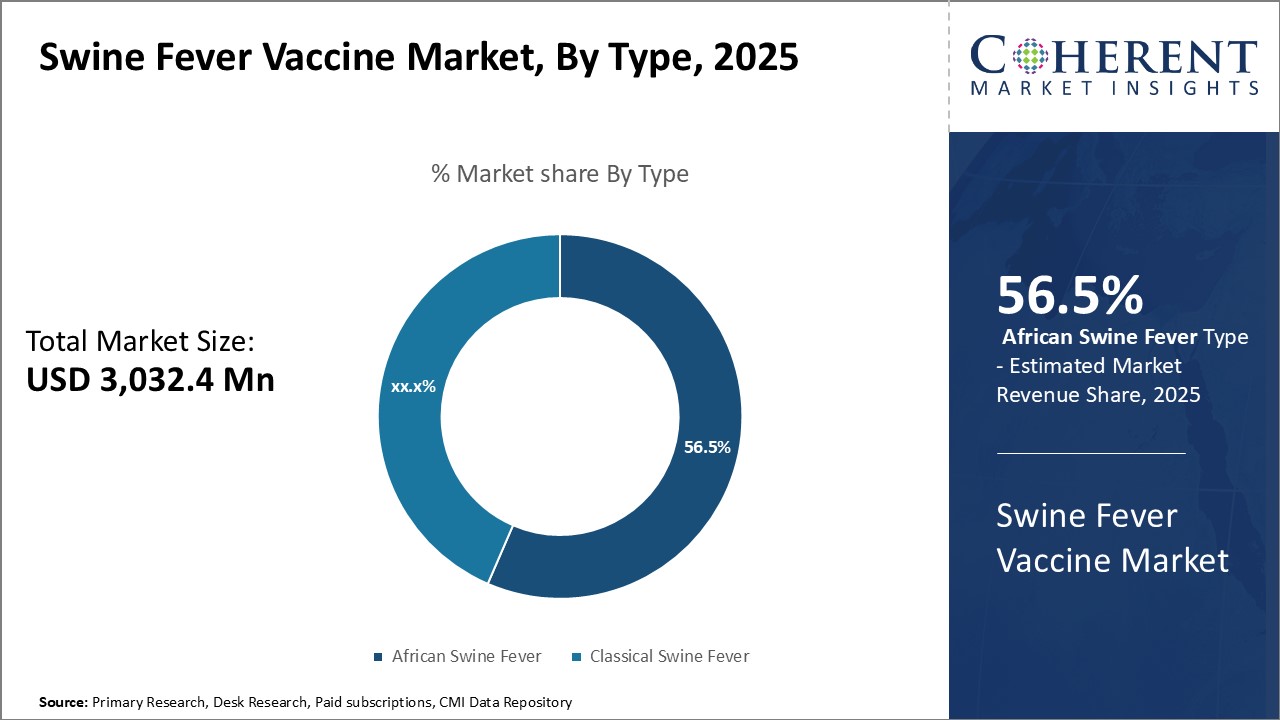
Figure 2. Global Swine Fever Vaccine Market Share, By Type, 2025
To learn more about this report, Download Free Sample
Recent Developments
In May 2022, Boehringer Ingelheim, a global leading company in animal health, announced its latest innovation: TwistPak, a revolutionary mixing platform. It enables swine producers to combine two vaccines, Ingelvac CircoFLEX and Ingelvac MycoFLEX, in a convenient, fast and flexible way.
In June 2020, Boehringer Ingelheim announced that it’s China-developed Classical Swine Fever Vaccine, Live (C-strain, PK/WRL Cell Line Origin) (English commercial name: Ingelvac CSF MLV) has just received a "New Veterinary Drug Registration Certificate" from the Ministry of Agriculture and Rural Affairs of China. It is the first Classical Swine Fever live vaccination co-developed by a foreign corporation and Chinese research organizations.
Increasing research and development activities by government and non-government organizations and key players in the market are expected to drive the market growth during the forecast period.
Increasing efforts by the governments of various countries and key players in the market to develop swine fever vaccines are expected to drive the market growth over the forecast period. For instance, in June 2022, Vietnam had developed an African swine fever vaccine, NAVET-ASFVAC, for pigs in partnership with the U.S. The Vietnamese researchers worked with the U.S. scientists on the NAVET-ASFVAC jab since 2020.
Global Swine Fever Vaccine Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
The coronavirus pandemic had negatively impacted the development, production, and supply of medicines and the growth of the pharmaceutical businesses of various companies across the globe. The lockdown has resulted in the closure of industrial establishments, except for the manufacturing of essential commodities, and there has been a disruption in the supply chain of pharmaceuticals. Thus, the COVID-19 pandemic had affected the economy in three main ways: 1) by directly affecting the production and demand; 2) disrupting distribution channels; 3) causing a financial impact on firms and financial markets.
Supply chain and manufacturing activities in India, China, and the U.S. were disrupted due to the global lockdown, while many countries such as Saudi Arabia, the UAE, Egypt, and others are facing problems with regard to the transportation of drugs and vaccines from one place to another. Due to all these reasons, the growth of the global swine fever vaccine market was negatively hampered.
Global Swine Fever Vaccine Market: Restraint
The major factors that hinder the growth of the global swine fever vaccine market include stringent regulatory approvals. The federal agencies involved in the approval of animal medicines are the United States Department of Agriculture (USDA) (biologics such as vaccines), the U.S. Food and Drug Administration (pharmaceuticals), and the Environmental Protection Agency (EPA) (pesticides such as flea and tick medicine). While undergoing approval, animal medicines are vigorously researched and tested for safety, purity, and efficacy, a process that can take five to seven years and cost tens of millions of dollars. Thus, stringent regulatory approvals may act as a restraint for the market growth.
Key Players
Major players operating in the global swine fever vaccine market include Merck & Co., Inc. (Merck Animal Health), Ceva, Zoetis Services LLC, Boehringer Ingelheim International GmbH, Indian Immunologicals Ltd., Bioveta, a.s., Komipharm, and EC21 Inc.
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients