Subcutaneous Biologics Market Size and Forecast – 2026 – 2033
The Global Subcutaneous Biologics Market size is estimated to be valued at USD 44.8 billion in 2026 and is expected to reach USD 78.9 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 8.2% from 2026 to 2033.
Global Subcutaneous Biologics Market Overview
This market is experiencing steady growth, driven by rising prevalence of chronic diseases, increasing adoption of self-administered therapies, and a shift from intravenous to subcutaneous delivery for improved patient convenience. Key therapeutic areas include oncology, autoimmune disorders, and rare diseases, where biologics offer targeted treatment options. Technological advancements in formulation and delivery devices, such as prefilled syringes and auto-injectors, are enhancing safety, efficacy, and patient adherence. North America dominates the market, supported by advanced healthcare infrastructure and reimbursement policies, while Asia Pacific demonstrates the fastest growth due to expanding healthcare access, government initiatives, and rising awareness of biologic therapies.
Key Takeaways
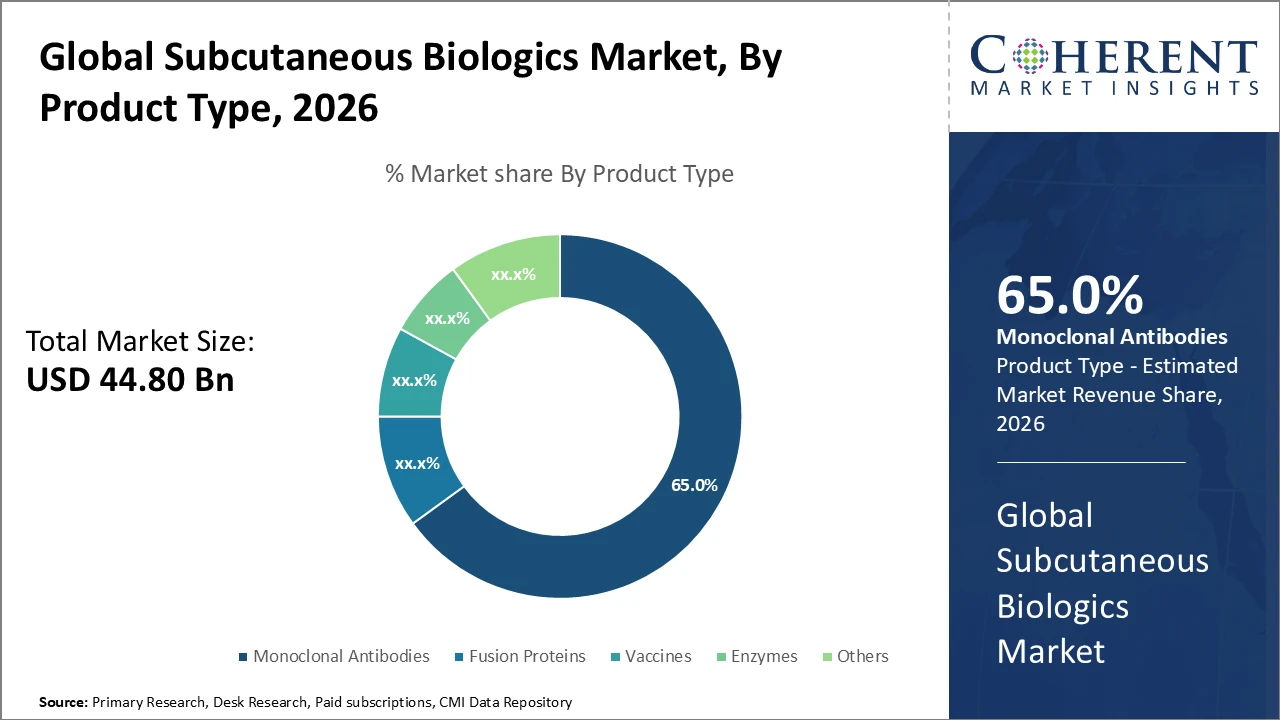
• The monoclonal antibodies segment dominates the subcutaneous biologics market, driven by extensive applications in oncology and autoimmune therapies, accounting for 65% of industry share.
• Autoimmune diseases remain the largest therapeutic area, representing nearly 47% of market revenue, highlighting the need for targeted biologic interventions.
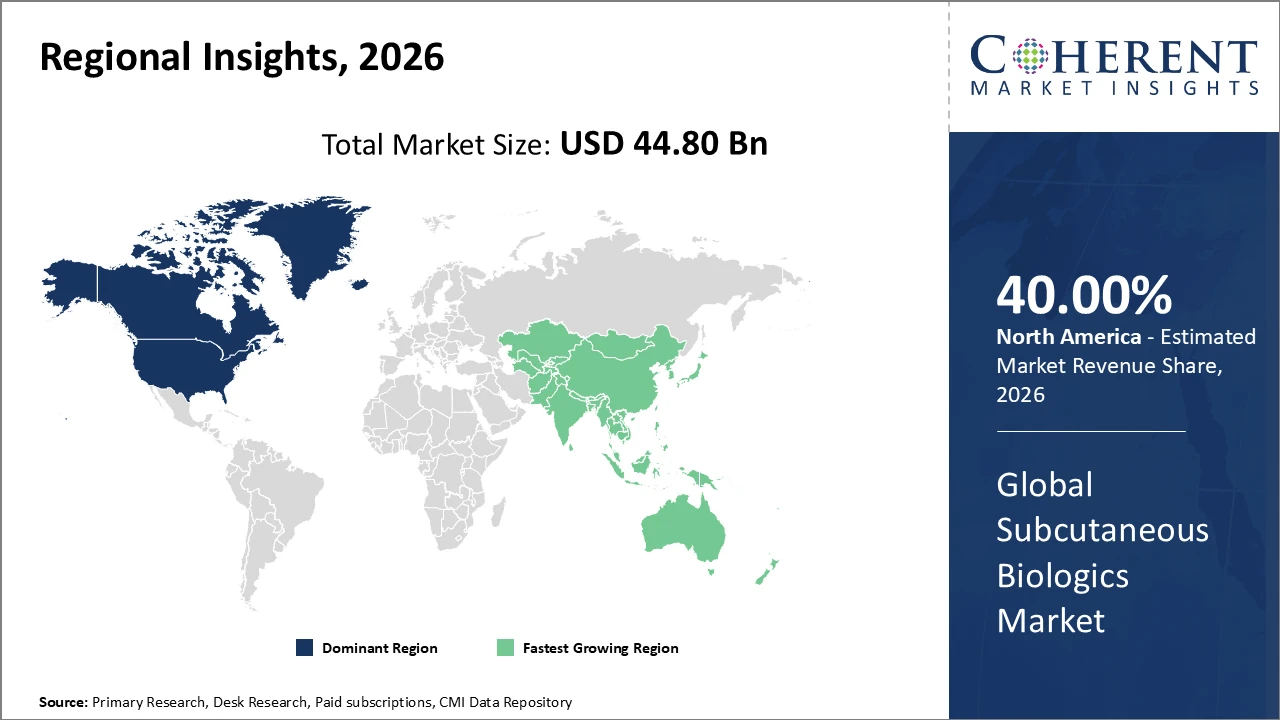
• North America holds the largest regional share, over 40%, supported by advanced healthcare infrastructure and significant R&D investment.
• Asia Pacific is the fastest-growing market, with a CAGR exceeding 10%, fuelled by increasing healthcare access and government initiatives promoting biologics.
Subcutaneous Biologics Market Segmentation Analysis
To learn more about this report, Download Free Sample
Subcutaneous Biologics Market Insights, By Product Type
Monoclonal antibodies dominate the subcutaneous biologics market with 65% share in 2026, driven by their broad therapeutic applications in oncology and autoimmune diseases, supported by substantial R&D investments and strong patent portfolios. The fastest-growing subsegment, fusion proteins, is benefiting from advances in molecular engineering that enhance efficacy and reduce immunogenicity, leading to wider clinical adoption.
Vaccines and enzymes contribute to market diversification, with emerging applications in infectious diseases and rare disorders. Other biologic modalities, including peptides, are gradually gaining clinical consideration, reflecting the industry’s ongoing innovation and expansion into new therapeutic areas.
Subcutaneous Biologics Market Insights, By End-User
Hospitals command the largest share of the subcutaneous biologics market, owing to their infrastructure and capability to administer complex biologic therapies. Homecare settings represent the fastest-growing segment, driven by the ease of self-administration with auto-injectors and cost-containment strategies that favour outpatient treatment models. Clinics contribute through specialized outpatient care services, while the ‘Others’ category, including nursing homes and specialty pharmacy providers, is gradually expanding access to biologics and supporting broader market adoption.
Subcutaneous Biologics Market Insights, By Therapeutic Area
Autoimmune diseases dominate the subcutaneous biologics market, driven by the high prevalence of conditions such as rheumatoid arthritis and psoriasis, along with established treatment protocols involving subcutaneous biologics. Oncology is the fastest-growing segment, fueled by breakthrough therapies including checkpoint inhibitors and monoclonal antibodies targeting specific cancer antigens. Infectious diseases experience steady growth supported by vaccination programs and antiviral subcutaneous biologics, while metabolic disorders and other therapeutic areas maintain a smaller yet consistent share through specialized niche treatments.
Subcutaneous Biologics Market Trends
• The subcutaneous biologics market is shifting toward self-administration therapies supported by intuitive delivery devices integrated with digital monitoring, enhancing real-time patient adherence data.
• In 2026, smart auto-injectors equipped with Bluetooth technology accounted for over 22% of new product launches, indicating a clear market shift toward connected devices.
• AI and machine learning integration in formulation development has accelerated the identification of stable subcutaneous biologics, reducing R&D cycle times by 15%.
• Emerging combination therapies delivered subcutaneously are targeting multifactorial diseases, supported by a 10% increase in clinical trial success rates in 2025.
Subcutaneous Biologics Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Subcutaneous Biologics Market Analysis and Trends
In North America, the subcutaneous biologics market is dominated by a well-established healthcare ecosystem, significant investment in biologic R&D, and supportive regulatory policies that expedite product approvals. The U.S. plays a central role, accounting for over 40% of the market, driven by leading companies such as Amgen and AbbVie, which maintain substantial manufacturing capabilities and innovation-focused operations.
Asia Pacific Subcutaneous Biologics Market Analysis and Trends
Asia Pacific is experiencing the fastest growth in the subcutaneous biologics market, with a CAGR exceeding 10%, driven by a rising incidence of chronic diseases, expanding healthcare infrastructure, and government initiatives promoting biosimilar adoption. Countries such as China and India are leading this expansion through local manufacturing investments and regulatory reforms that support the development and commercialization of biopharmaceuticals.
Subcutaneous Biologics Market Outlook for Key Countries
USA Subcutaneous Biologics Market Analysis and Trends
The U.S. subcutaneous biologics market is marked by intense innovation, with companies like Amgen and Roche leading pipeline developments and expanding production capacities. Recent FDA fast-track approvals of biosimilars in 2025 have accelerated market penetration, while extensive insurance coverage has improved patient access. High healthcare expenditure, combined with well-established clinical research infrastructure, enables the U.S. to contribute significantly to global market revenue.
Germany Subcutaneous Biologics Market Analysis and Trends
Germany’s subcutaneous biologics market is growing steadily, driven by a robust healthcare infrastructure, strong pharmaceutical R&D, and supportive government strategies that streamline approvals and encourage local manufacturing. Favorable reimbursement policies and physician preference for advanced biologic therapies, including subcutaneous immunoglobulins, enhance adoption across hospitals and clinics.
Increasing emphasis on home-based care and self-administration options improves patient compliance and reduces hospital burden. Technological advancements in delivery devices, coupled with R&D collaborations among leading biopharma companies, further strengthen market momentum. Regulatory support for biosimilars through the European Medicines Agency also expands access, promotes competitive pricing, and reinforces Germany’s role as a biopharma hub.
Analyst Opinion
• The surge in biosimilar approvals is reshaping market share, with biosimilars accounting for approximately 35% of subcutaneous biologics market revenue in 2025, reflecting intensified competition and increased accessibility. This indicates a shift toward cost-effective treatment alternatives in both emerging and established markets.
• Producer-side growth is driven by expanded production capacity through advanced bioprocessing technologies. Between 2024 and 2026, manufacturers increased subcutaneous biologic output by nearly 20%, enhancing supply reliability and market penetration.
• On the demand side, pricing dynamics and growing patient populations stimulate revenue. In 2025, subcutaneous biologics maintained 12% price stabilization despite broader inflation, making them more competitive than traditional intravenous formulations.
• Micro-level adoption trends show diversification in use cases. While autoimmune diseases remain the largest therapeutic category, oncology-related subcutaneous biologics usage surged by 15% in 2026 due to improved formulation stability and patient preference, signalling a pivotal shift in clinical protocols.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 44.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 8.2% | 2033 Value Projection: | USD 78.9 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Amgen Inc., Roche Holding AG, Sanofi S.A., AbbeVie Inc., Johnson & Johnson, Novo Nordisk A/S, Pfizer Inc, Biogen Inc., Merck & Co Inc., CSL Behring | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Subcutaneous Biologics Market Growth Factors
The expanding prevalence of chronic and autoimmune diseases continues to drive growth in the subcutaneous biologics market, with rheumatoid arthritis affecting nearly 1.5 million people in the U.S. alone in 2025. Innovations in drug delivery devices, including auto-injectors and wearable patches, have improved patient adherence, evidenced by an 18% increase in home administration of subcutaneous biologics in 2026. Favorable regulatory frameworks in North America and Europe have fast-tracked approvals of novel subcutaneous biologics, reducing time-to-market by up to six months. Additionally, rising healthcare expenditure, with global biologics revenue increasing 9% in 2025, supports sustained market expansion.
Subcutaneous Biologics Market Development
In January 2026, Biogen and Eisai received FDA approval in November 2025 for a subcutaneous formulation of Leqembi (lecanemab), an anti-amyloid beta monoclonal antibody for Alzheimer’s disease. This biologics license application enables easier administration compared to intravenous infusion, reducing treatment burden and enhancing patient convenience while expanding access to ongoing therapy.
In December 2024, the subcutaneous biologics market saw significant activity across approvals, pipelines, and strategic developments. The FDA approved a subcutaneous formulation of nivolumab with hyaluronidase for solid tumours, expanding convenient cancer therapy options. Johnson & Johnson submitted a BLA for a subcutaneous combination therapy targeting non‑small cell lung cancer. Takeda received U.S. FDA approval for subcutaneous ENTYVIO for Crohn’s disease, reflecting expanding indications. OneSource Specialty Pharma entered a strategic licensing deal for high‑concentration subcutaneous delivery technology. Across the year, investment in advanced delivery platforms and partnerships to enhance patient‑friendly biologics underscored growing market momentum and diversification.
Key Players
Leading Companies of the Market
Amgen Inc
Roche Holding AG
Sanofi S.A.
AbbVie Inc.
Johnson & Jhonson
Novo Nordisk A/S
Pfizer Inc.
Biogen Inc.
Merck & Co. Inc.
CSL Behring
Market players in the subcutaneous biologics industry are leveraging acquisitions and strategic partnerships to strengthen their portfolios and manufacturing capabilities. In 2025, a leading biopharmaceutical company acquired a contract manufacturing organization specializing in subcutaneous biologics, achieving a 25% increase in production efficiency within 12 months. Another company expanded its R&D initiatives by establishing a dedicated innovation center in 2026, accelerating the development of new subcutaneous formulations and enhancing its product pipeline. These strategies highlight the industry’s focus on operational efficiency, innovation, and faster market delivery.
Subcutaneous Biologics Market Future Outlook
The subcutaneous biologics market is poised for sustained growth driven by rising prevalence of chronic and autoimmune diseases, increasing patient preference for self-administered therapies, and innovations in delivery technologies such as auto-injectors and wearable devices. Expansion of biosimilars and combination therapies will enhance accessibility and cost-effectiveness, particularly in emerging markets. Regulatory support and favorable reimbursement policies in North America and Europe will accelerate adoption, while Asia Pacific is expected to witness the fastest growth due to improving healthcare infrastructure and local manufacturing initiatives. Ongoing R&D, digital monitoring integration, and patient-centric innovations will continue to shape market dynamics through 2033.
Subcutaneous Biologics Market Historical Analysis
The subcutaneous biologics market has evolved significantly over the past decade. Initially, adoption was slow due to limited self-administration options, moderate awareness, and reliance on intravenous formulations. Growth accelerated with the introduction of monoclonal antibodies and other subcutaneous biologics offering enhanced convenience and patient compliance. Technological innovations in delivery devices, such as prefilled syringes and auto-injectors, further boosted uptake. Increasing prevalence of chronic and autoimmune diseases, along with supportive regulatory frameworks in North America and Europe, contributed to steady market expansion. Over time, the emergence of biosimilars and combination therapies has diversified offerings and strengthened the market’s global presence.
Sources
Primary Research Interviews:
Biopharmaceutical R&D Scientists
Clinical Pharmacologists
Hospital Pharmacists and Physicians
Hospital Pharmacists and Physicians
Databases:
WHO Global Health Observatory
OECD Health Data
IQVIA Biopharmaceutical Market Reports
Magazines:
Pharmaceutical Technology
BioPharma Reporter
Drug Development & Delivery
Fierce Biotech
Journals:
Journal of Biotechnology
mAbs Journal
Biologicals
Vaccine
Pharmaceutical Research
Newspapers:
The New York Times (Health)
The Guardian (Health)
Financial Times (Healthcare)
The Hindu (Health)
Reuters Health
Associations:
World Health Organization (WHO)
International Society for Pharmaceutical Engineering (ISPE)
Biotechnology Innovation Organization (BIO)
European Federation of Pharmaceutical Industries and Associations (EFPIA)
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients