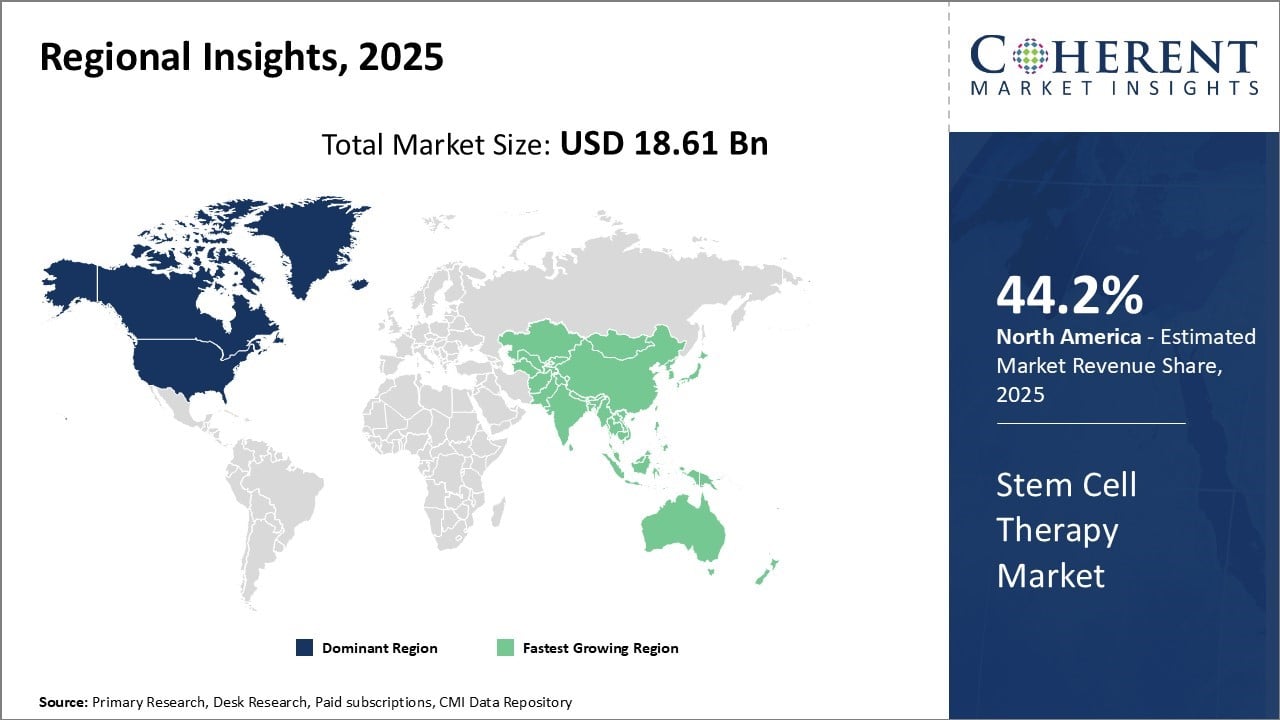
Global stem cell therapy market is estimated to be valued at USD 18.61 Bn in 2025 and is expected to reach USD 78.39 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 22.8% from 2025 to 2032.
To learn more about this report, Download Free Sample
The Stem Cell Therapy Market is witnessing remarkable expansion primarily due to the rising prevalence of chronic diseases and greater needs for regenerative medicine. In addition, stem cell treatments are being safely and effectively adopted around the world owing to advancements in biotechnology. For instance, the FDA’s approval of stem cell treatments for specific blood disorders substantially broadens their clinical use, strengthening market optimism. Moreover, the innovations are further fueled by the growing research and development spending by pharmaceutical companies. This is also illustrated by the rising adoption of stem cell therapy for the treatment of leukemia and osteoarthritis, which demonstrates its wider therapeutic use. Collectively, these factors suggest strong opportunities for growth in the stem cell therapy market in the coming years.
|
Event |
Description and Impact |
|
FDA and Global Regulatory Framework Evolution |
|
|
China’s Stem Cell Research and Clinical Trial Acceleration |
|
|
Breakthrough Technologies in Cell Manufacturing and Delivery |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The impact of artificial intelligence (AI) on stem cell therapy is profound, especially in the areas of research, manufacturing, and clinical outcomes. Machine learning technologies analyze vast datasets to anticipate stem cell activities, refine therapeutic pathways, and expedite the development of therapies.
As an example, the California Institute for Regenerative Medicine partners with AI companies to predict stem cell differentiation to save on costly and time-consuming experiments. In manufacturing, AI platforms enhance the differentiation of stem cells and improve quality control by monitoring cellular morphology and gene expression to identify anomalies that may compromise the treatment's efficacy.
Fate Therapeutics uses AI to engineer induced pluripotent stem cells (iPSCs) for targeted cancer immunotherapies, enabling the iPSC-derived cell therapies to be produced as off-the-shelf products. AI is also embedded in Celularity's placental stem cell platform for the selection of therapeutics and protocol refinement, enabling a 40% acceleration in clinical trial timelines and optimizing patient matching.
AI in stem cell therapy provides enhanced treatment personalization and precision for patients and reliable therapeutic outcomes for practitioners. In summary, AI application in stem cell therapy is transforming the field of regenerative medicine by providing precision at lower costs and increasing the industry-wide scalability and efficacy of the treatments.
The global Stem Cell Therapy Market is expanding rapidly, with treatments for conditions such as Autism Spectrum Disorder, COPD, Liver Cirrhosis, Eye Disorders, and Erectile Dysfunction varying widely in cost. Pricing is influenced by therapy complexity, cell source, regulatory status, and treatment setting.
For example, Mesenchymal Stem Cell (MSC) therapies—commonly used for conditions like liver cirrhosis and eye disorders—range from $5,000 to $25,000 per treatment depending on whether the cells are adipose, bone marrow, or umbilical cord-derived. Autologous MSC treatments tend to be less expensive but vary based on processing needs.
The expense of advanced manufacturing and regulatory approvals reflects in the price of more complex therapies like gene-modified CAR-T cell therapies, which can exceed $400,000. Stem cell therapies aimed at patients suffering from COPD and erectile dysfunction are more mid-tier in price, striking a balance between effectiveness and manufacturing costs. There are also price differentials based on geographical location and customer, with prices for services done in a hospital often higher than those done in a clinic.
Although prices are high, there is increasing clinical evidence and a strong demand from patients, which is slowly but surely changing insurance coverage and reimbursement policies aimed to enhance affordability in the burgeoning market for stem cell therapy.
Insights from important stakeholders in the stem cell therapy ecosystem spanning IVF Clinics, pharmaceutical and biotechnological companies, academic and research institutions, and biobanks illustrate varying opinions regarding the stem cell therapy market.
To learn more about this report, Download Free Sample
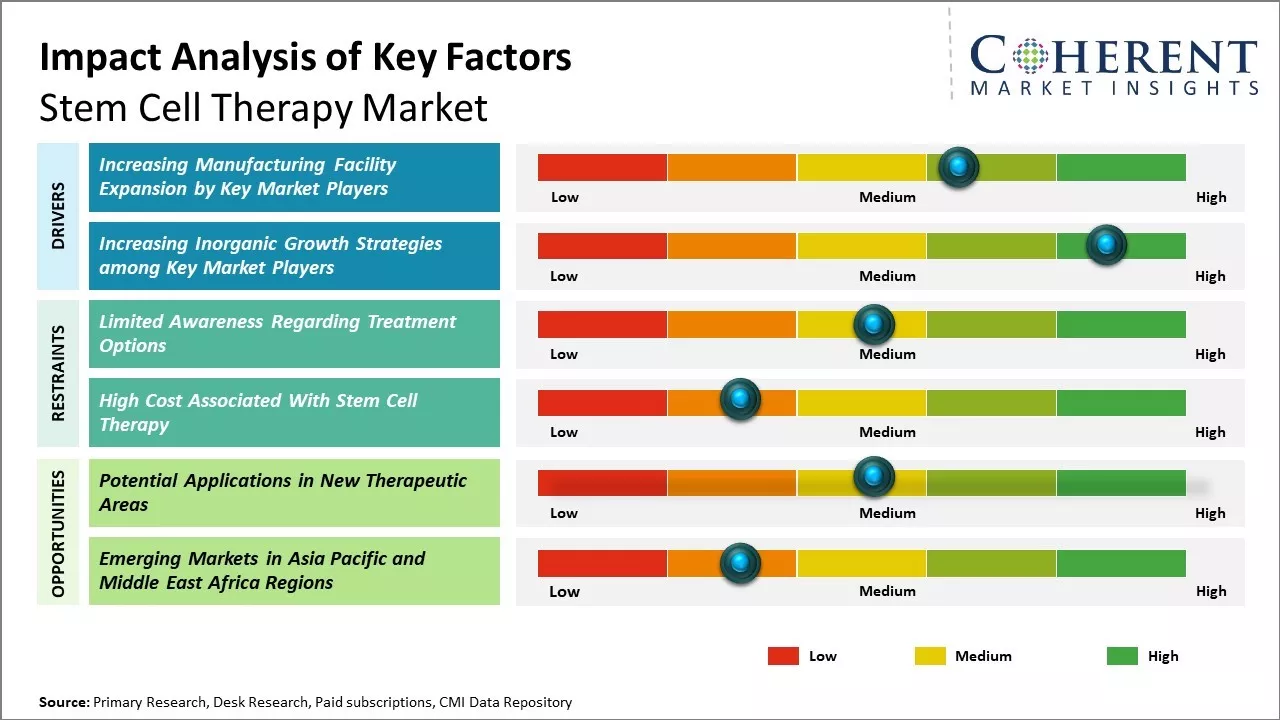
Increasing adoption of inorganic growth strategies such as partnerships by key market players is expected to drive the market growth over the forecast period. For instance, in June 2022, The Melbourne node of the Novo Nordisk Foundation Center for Stem Cell Medicine (reNEW) announced a partnership with Murdoch Children's, the University of Copenhagen, and Leiden University Medical Center in the Netherlands, for stem cell research that will develop new treatments for cancer, diabetes and heart disease has been launched at the Murdoch Children’s Research Centre (MCRI).
Limited awareness regarding treatment options and the ethical concerns related to embryonic stem cells can hamper the market growth. For instance, according to the data published by the National Center for Biotechnology Information, there is main factor concerning is the fear of the unknown therapy and lack of awareness among the population about the treatment options. Moreover, high cost for these therapies leads to lower adoption, thus, leading to reduced demand for the product worldwide.
Potential applications in new therapeutic areas can offer significant opportunities for growth of global stem cell therapy market. Stem cell therapy holds promise for treating various diseases that currently have limited therapeutic options. Researchers worldwide are engaged in developing novel stem cell-based treatments for conditions such as neurodegenerative disorders, cardiovascular diseases and diabetes. According to World Health Organization, stroke is a leading cause of disability and the second leading cause of death worldwide.
Cell therapy using stem cells is being investigated for its potential to treat stroke and regenerate damaged brain tissue. Several clinical studies are underway evaluating the safety and efficacy of stem cells for ischemic stroke. Promising results from early studies suggest stem cells may improve neurological outcomes when administered shortly after a stroke.
Continued research aims to enhance the therapeutic potential for conditions with high unmet needs like stroke. As research expands into new areas, it is expected to drive significant growth of global stem cell therapy market. Continuous technological advancements to enhance the therapeutic potential of stem cells across different disease areas will promote their clinical adoption. This will pave the way for stem cell-based treatments to benefit millions suffering from diseases lacking effective cures.
Cell Source segment is sub-segmented adult stem cells, induced pluripotent stem cells, embryonic stem cells, and others. Adult stem cells segment is estimated to hold 44.2% of the market share in 2025 owing to significant advancements in isolation techniques. Adult stem cells can be extracted from various tissues such as bone marrow, blood, skin and muscles. Unlike embryonic stem cells, their usage does not involve any ethical issues.
Moreover, adult stem cells are more specialized than embryonic stem cells and can differentiate into a smaller number of cell types. Continuous research over the past decade has led to refined isolation protocols for extracting adult stem cells with high purity levels from different sources, and this has augmented their therapeutic applications. Banked adult stem cells can also be utilized for allogeneic transplantations, if matching tissue or cell types are available.
Application segment is sub-segmented musculoskeletal disorders, wounds and injuries, cancer, autoimmune disorders, and others. Musculoskeletal disorders segment is estimated to hold 39.7% of the market share in 2025, owing to growing prevalence of conditions such as osteoarthritis and bone fractures globally.
Skeletal applications of stem cells including cartilage and bone regeneration are gaining popularity due to advantages such as enhanced recovery time, donor site morbidity reduction and ability of stem cells to augment the healing process. With ageing demographics and rising incidence of osteoporosis in developed nations, there will be increase in demand for stem cell therapies in orthopedics.
End User segment is sub-segmented into hospitals, cell banks, academic and research institutes. Hospitals segment is estimated to hold 33.1% of the market share in 2025 due to existing reimbursement policies and clinical expertise for stem cell procedures. While cell therapy products are still under regulatory approvals, several medical insurance plans provide partial to full coverage for procedures performed in hospitals. This makes stem cell treatment affordable for a larger patient base.
Moreover, trained paramedics, sterile facilities and emergency care provisions allow hospitals to minimize procedure-related risks. Cell expansion and processing expertise also enables translation of novel stem cell therapies developed in research institutes to clinical settings. Rising popularity of outpatient clinics and day care centers for stem cell administration is anticipated to supplement hospitals’ share in the near future. However, regulatory hurdles continue to limit stem cell applications to third-party commercial settings.
To learn more about this report, Download Free Sample
North America continues to hold a dominant position in the global stem cell therapy market, with an estimated 44.2% market share in 2025. The region’s stronghold is driven primarily by the U.S. and Canada, where a robust biotechnology and pharmaceutical industry fuels significant investments in stem cell research and development.
A well-established healthcare infrastructure and supportive regulatory environment—highlighted by the U.S. FDA’s approvals of several stem cell therapies—have enabled widespread clinical adoption. Additionally, the availability of private and public insurance coverage for stem cell therapy procedures has further accelerated market growth in this region. These factors collectively position North America as the leading market for advanced stem cell therapies globally.
Asia Pacific has emerged as the fastest growing market in the global stem cell therapy landscape. Rapid improvements in healthcare infrastructure, a surge in medical tourism, and proactive government policies in countries like China, India, South Korea, and Japan are driving this growth. The region has become a hub for affordable, high-quality stem cell therapies, supported by state-of-the-art stem cell banks and therapy centers offering cost-effective treatment options compared to Western markets.
Medical tourism plays a key role, attracting international patients seeking therapies not available or approved in their home countries. Governments actively support the sector through incentives to boost private investments and enhance domestic production.
South Korea’s leadership in umbilical cord blood banking, combined with stringent quality regulations and international accreditation of clinics, underscores the region’s potential. This unique convergence of advanced research capabilities and affordable treatment delivery makes Asia Pacific a critical growth engine in the global stem cell therapy market.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 18.61 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 22.8% | 2032 Value Projection: | USD 78.39 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
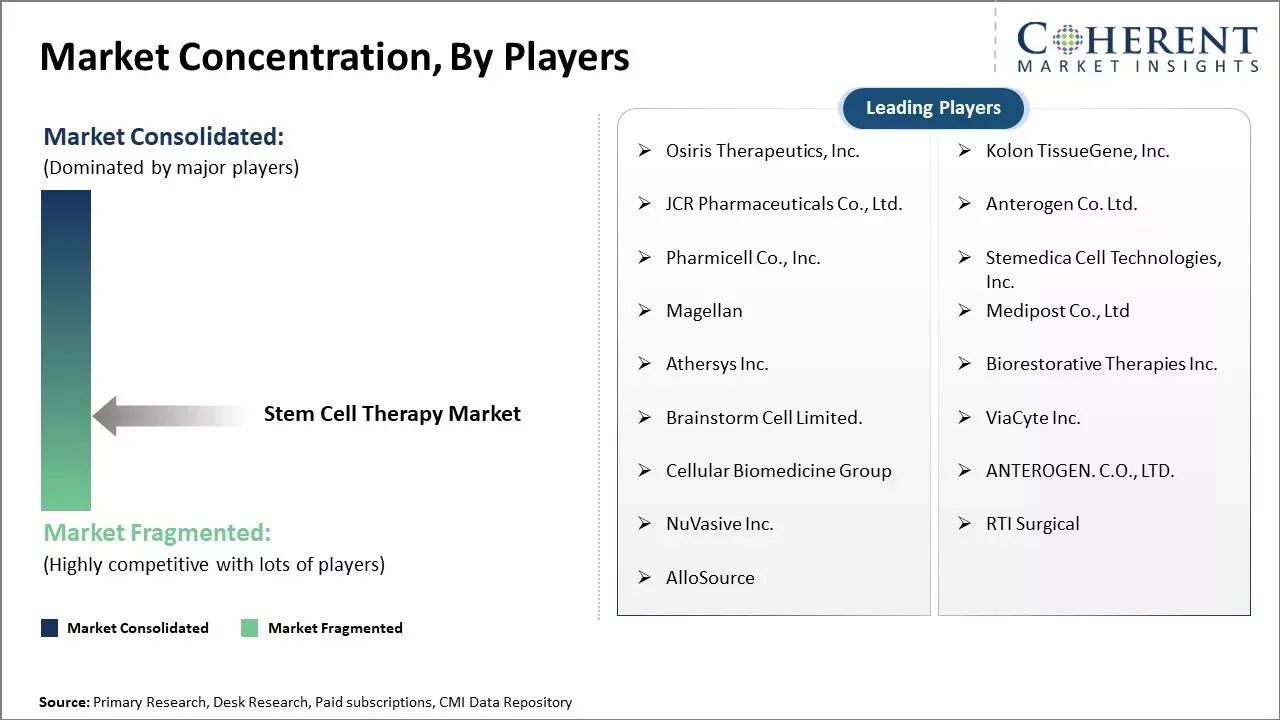
Osiris Therapeutics, Inc., Kolon TissueGene, Inc., JCR Pharmaceuticals Co., Ltd., Anterogen Co. Ltd., Pharmicell Co., Inc., Stemedica Cell Technologies, Inc., Magellan, Medipost Co., Ltd, Athersys Inc., Biorestorative Therapies Inc., Brainstorm Cell Limited., ViaCyte Inc., Cellular Biomedicine Group, ANTEROGEN. C.O., LTD., NuVasive Inc., RTI Surgical, AlloSource |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: Stem cell therapy, also known as regenerative medicine, promotes the repair response of diseased, dysfunctional or injured tissue using stem cells or their derivatives. It is the next chapter in organ transplantation and uses cells instead of donor organs, which are limited in supply. Stem cells are cells with the potential to develop into many different types of cells in the body. These serve as a repair system for the body. There are two main types of stem cells embryonic stem cells and adult stem cells. Stem cells are divided into two major classes; pluripotent and multipotent. Pluripotent stem cells are replicating cells, which are derived from the embryo or fetal tissues. The pluripotent stem cells facilitate the development of cells and tissues in three primary germ layers such as mesoderm, ectoderm, and endoderm.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients