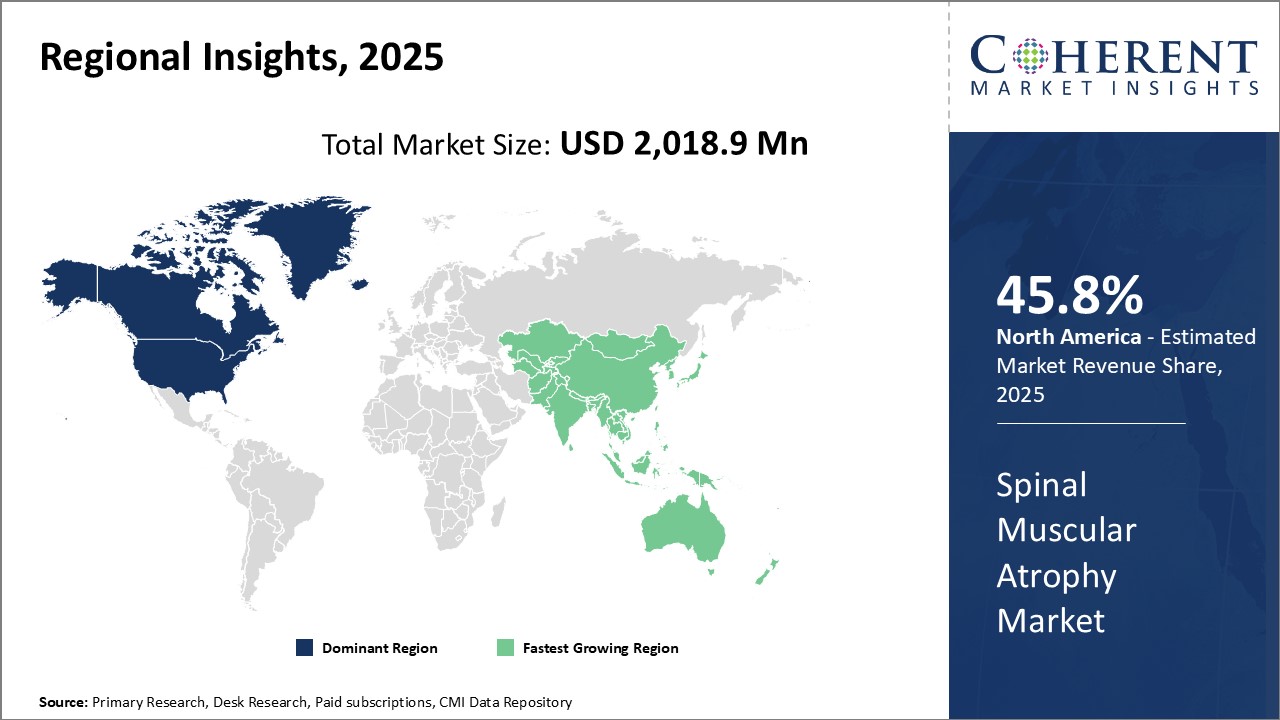
Global spinal muscular atrophy market size is estimated to be valued at USD 2,018.9 Mn in 2025 and is expected to exhibit a CAGR of 13.5% during the forecast period (2025-2032).
Spinal Muscular Atrophy (SMA) is characterized as a genetic condition with gradual muscle motor weakness as a result of loss of motor neurons, affecting firstly the muscles closest to the center of the body. The spinal muscular atrophy market growth is expanding rapidly with the introduction of new gene therapies like Zolgensma by Novartis, which has been approved by the FDA, and aims to treat the genetic cause of the disease. Broader diagnosis initiatives, growing concern, and favorable reimbursement policies are propelling growth in the SMA market. Active studies, along with increasing healthcare availability in developing areas, heighten overall growth, positioning SMA as a key focus in the therapeutics of rare diseases.
|
Event |
Description and Impact |
|
Advances in Gene Therapy Research |
|
|
Global Pharmaceutical Industry Supply Chain Disruptions |
|
|
Economic Instability and Healthcare Budget Constraints |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The Type 1 segment is expected to dominate the spinal muscular atrophy market with a commanding share of 57.2% in 2025. This dominance is primarily due to the severe nature and early onset of Type 1 SMA, also known as infantile-onset SMA, which necessitates immediate and intensive therapeutic intervention. The high prevalence and critical need for effective treatments in newborns and infants drive significant demand for advanced therapies targeting this segment.
Patients with Type 1 SMA experience rapid motor neuron degeneration leading to severe muscle weakness and respiratory complications, making early diagnosis and treatment crucial. Consequently, pharmaceutical advancements focusing on gene therapy, antisense oligonucleotides, and supportive care are predominantly tailored for this segment, fueling market growth.
To learn more about this report, Download Free Sample
North America is projected to dominate the global spinal muscular atrophy market, accounting for an estimated 45.8% share in 2025. This leadership position is driven by the region’s advanced healthcare infrastructure, strong reimbursement frameworks, and widespread awareness about genetic disorders. The U.S. and Canada host numerous specialized treatment centers and research institutions focused on rare neuromuscular diseases, facilitating early diagnosis and access to innovative therapies.
Robust government and private funding for genetic research and rare disease drug development further strengthen the region’s competitive edge. Additionally, comprehensive insurance coverage and patient support programs ensure better affordability and adherence to high-cost SMA treatments. North America’s collaborative ecosystem involving pharmaceutical companies, healthcare providers, and patient advocacy groups continues to propel market growth and drive breakthroughs in spinal muscular atrophy care.
Asia Pacific is anticipated to be the fastest-growing Spinal Muscular Atrophy Market Trends during the forecast period. Rapid economic growth, improving healthcare access, and increasing awareness about genetic conditions are key factors propelling demand in countries such as China, India, Japan, and South Korea. The expanding healthcare infrastructure and rising government initiatives focused on rare diseases are fostering enhanced diagnosis and treatment availability.
The region’s large patient pool and increasing adoption of advanced genetic therapies are attracting investments from global pharmaceutical companies aiming to capture growth opportunities. Moreover, cost-effective manufacturing and distribution channels, coupled with growing patient advocacy, are expected to sustain long-term market expansion in the Asia Pacific region.
The United States continues to lead the North American spinal muscular atrophy market, supported by its world-class healthcare system and extensive rare disease research initiatives. The presencae of leading biopharmaceutical companies specializing in SMA therapies, such as gene therapy and antisense oligonucleotides, underpins the country’s market dominance. Strong regulatory support from the FDA and expansive insurance coverage facilitate faster patient access to innovative treatments. Additionally, growing awareness campaigns and patient advocacy organizations contribute to improved diagnosis rates and early intervention, sustaining robust market growth.
China is emerging as a high-growth market within the Asia Pacific region, driven by increasing government focus on rare disease management and expanding healthcare infrastructure. The country’s large patient population, coupled with rising investments in genetic research and biotechnology, is accelerating the availability and adoption of advanced SMA therapies. Favorable policy reforms aimed at improving drug accessibility and reimbursement are also enhancing market prospects.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2,018.9 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 13.5% | 2032 Value Projection: | USD 4,898.9 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Biogen, CYTOKINETICS, F. Hoffmann-La Roche Ltd, Genentech, Inc., PTC Therapeutics, Inc., Novartis AG, Ionis Pharmaceuticals, Chugai Pharmaceutical Co., Ltd., NMD PHARMA A/S, and Astellas Pharma Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The increasing prevalence of spinal muscular atrophy (SMA) is expected to drive the market growth over the forecast period. For instance, according to the Spinal Muscular Atrophy Foundation, a voluntary organization whose mission is to accelerate the development of a treatment for spinal muscular atrophy, SMA is believed to affect around 10,000 to 25,000 children and adults in the U.S., and therefore it is one of the most common rare diseases.
Increasing product launches are expected to drive the market growth over the forecast period. For instance, in June 2021, F. Hoffmann-La Roche Ltd, a multinational healthcare company, launched prescription medicine Evrysdi, indicated for the treatment of spinal muscular atrophy (SMA) in adults and children aged 2 months and older, in India.
The major factor that hinder growth of the global spinal muscular atrophy market include high cost of the treatment. For instance, according to an article published by MJH Life Sciences, largest privately held medical media company in the U.S., in March 2021, the mean per-patient annual direct medical cost was estimated to be between US$ 3,320 (spinal muscular atrophy type 3) in Italy and US$ 324,210 (type 1) in the U.S., with the variability high for other measures as well.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients