Skin Replacement Market Size and Forecast – 2026 – 2033
The Global Skin Replacement Market size is estimated to be valued at USD 5.8 billion in 2026 and is expected to reach USD 11.3 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 10.2% from 2026 to 2033.
Global Skin Replacement Market Overview
Skin replacement products are advanced medical materials used to restore or replace damaged skin tissue. These include biological skin substitutes, synthetic grafts, and bioengineered skin constructs. Skin replacement products support wound healing by providing a protective barrier, promoting cell regeneration, and reducing infection risk. They are widely used in burn treatment, chronic wound care, trauma surgery, and reconstructive procedures. Biocompatibility, durability, and integration with host tissue are essential product attributes.
Key Takeaways
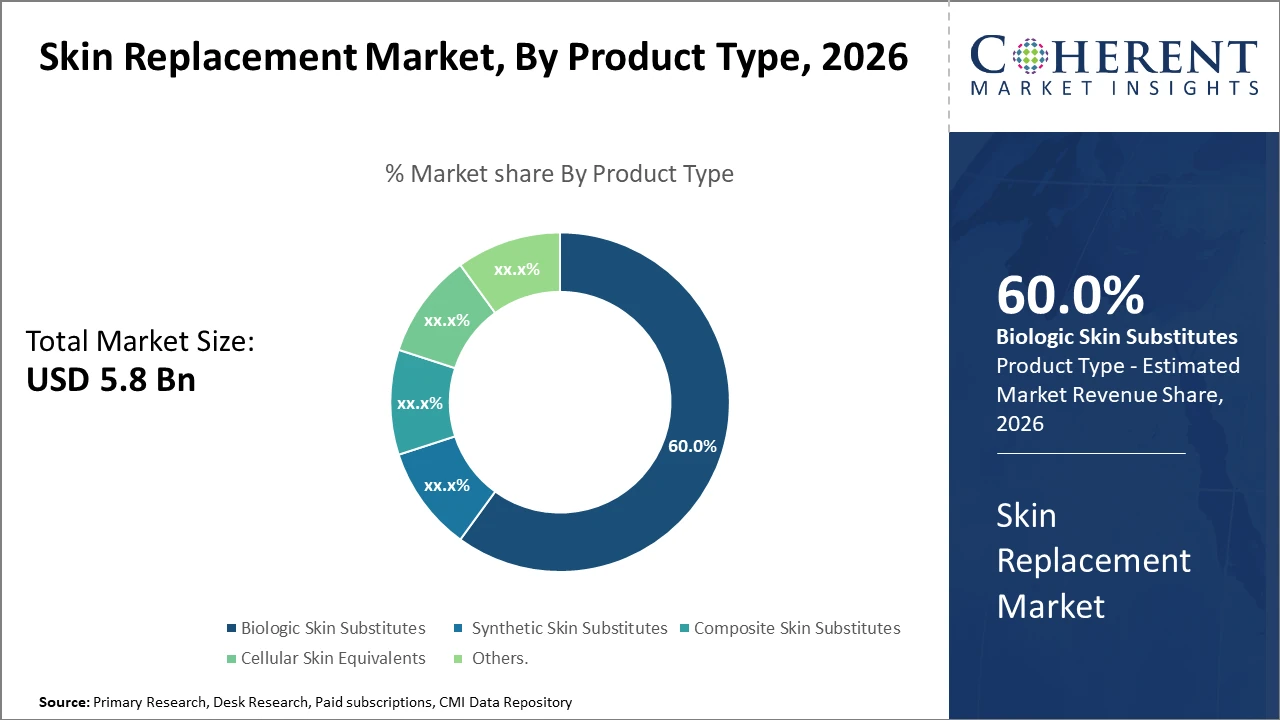
Biologic Skin Substitutes dominate with a 60% market share, propelled by their higher compatibility and integration into host tissue, while synthetic substitutes are the fastest growing due to cost-effectiveness and innovation in materials.
Burn treatment applications hold the largest industry share, accounting for nearly 45% of overall usage, but chronic wound management is growing faster, with a CAGR outpacing other segments due to aging populations and rising diabetes cases.
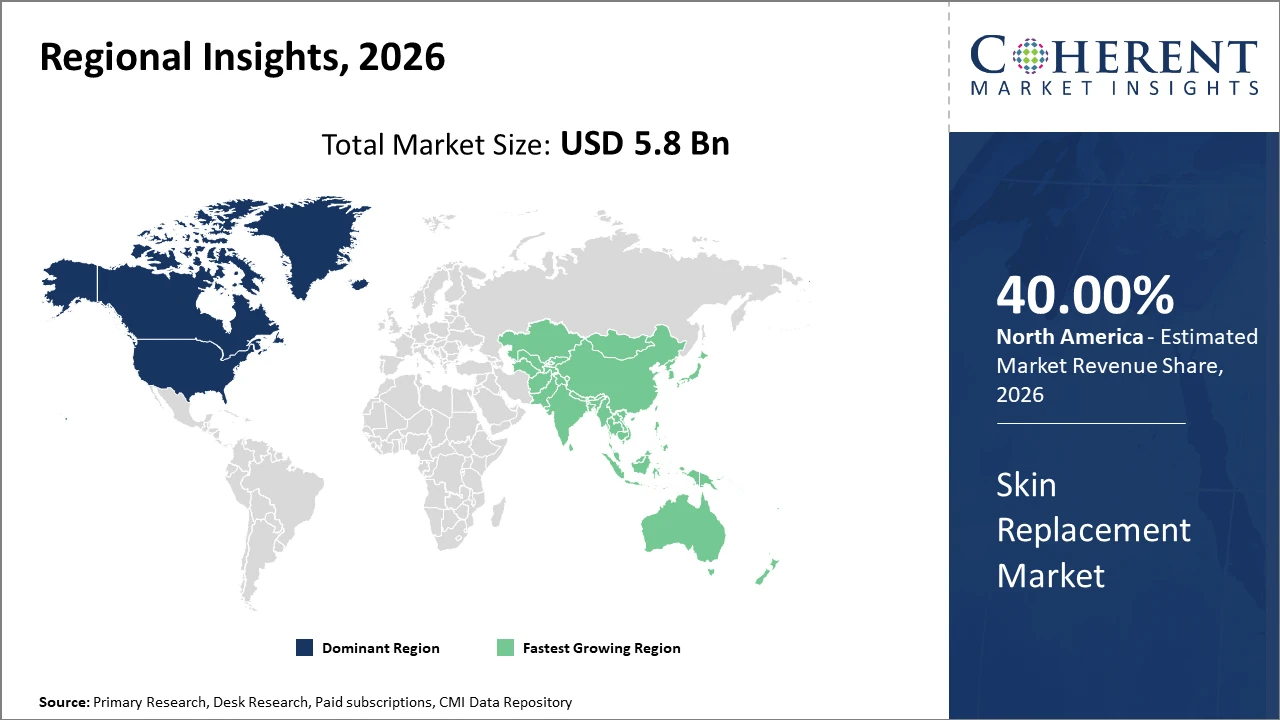
North America holds the dominant industry share by 40%, driven largely by the U.S., with robust healthcare infrastructure, reimbursement systems, and active market players contributing to 38% of the global market revenue.
Asia Pacific exhibits the fastest market growth, registering a CAGR exceeding 12%, fueled by expanding healthcare investments and government initiatives in countries like India and China to enhance wound care capabilities.
Skin Replacement Market Segmentation Analysis
To learn more about this report, Download Free Sample
Skin Replacement Market Insights, By Product Type
Biologic Skin Substitutes dominate the market share with 60%. Biologic substitutes are favored for their superior integration and reduced immunogenicity, making them pivotal in complex wound healing and reconstructive surgery. The fastest-growing subsegment is Synthetic Skin Substitutes, benefiting from innovations in polymers and materials science which have lowered costs and enhanced durability, thus fostering growth in cost-sensitive regions. Composite Skin Substitutes combine biologic and synthetic elements for hybrid solutions but currently hold a smaller market presence. Cellular Skin Equivalents leverage cultured cells for regenerative therapies but face challenges in scalability, limiting their widespread adoption. Other niche products include allografts and xenografts that serve specific clinical indications but with limited demand.
Skin Replacement Market Insights, By Application
Burn Treatment dominates the market share, commanding nearly 45%, due to the high incidence of burn injuries requiring immediate and effective skin regeneration. This segment benefits from ongoing product refinements focused on accelerating healing and minimizing scarring. The fastest-growing application is Chronic Wound Management, propelled by expanding diabetic and elderly populations and an increasing prevalence of pressure ulcers and venous leg ulcers globally. Surgical Reconstruction follows, driven by growing elective surgeries and trauma care requiring skin replacement solutions tailored to anatomical restoration. Cosmetic Procedures are gaining momentum slowly with rising aesthetic awareness but currently contribute modestly. Others encompass niche applications like veterinary dermatology and research use.
Skin Replacement Market Insights, By End-User
Hospitals dominate with the largest market share, as they offer comprehensive wound care services and have the infrastructure to utilize diverse skin replacement products. Hospitals remain central to complex cases such as major burns and surgical reconstruction. Specialty Clinics are the fastest-growing segment, driven by increasing outpatient treatment paradigms and focused expertise in wound management that demand innovative and quick-healing skin substitutes. Ambulatory Surgical Centers benefit from shorter hospital stays and cost containment, expanding their share as elective surgeries rise. Home Healthcare is an emerging segment, influenced by aging demographics and telemedicine facilitating remote chronic wound care, although it currently holds a smaller market portion.
Skin Replacement Market Trends
The market trend is heavily influenced by technological breakthroughs and evolving healthcare frameworks.
The surge in chronic wound incidence owing to global diabetes expansion propels demand for advanced skin substitutes, as evidenced by a 15% increase in prescription rates of bioengineered skin products in 2024.
The adoption of hybrid skin substitutes combining synthetic and biologic components is gaining traction due to improved healing efficacy and reduced immunogenicity.
Additionally, integration of AI and machine learning analytics in wound assessment is creating personalized treatment protocols, demonstrated by pilot programs in North America that reduced healing time by up to 20%.
Skin Replacement Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Skin Replacement Market Analysis and Trends
In North America, the dominance in the market is attributed to its mature healthcare ecosystem, significant R&D investments, and comprehensive reimbursement policies. The U.S. accounts for a vast majority of regional market revenue (38% global share), supported by companies like Mölnlycke Health Care and Integra LifeSciences driving innovation and adoption. Advanced clinical infrastructure and high patient awareness further consolidate the region's leadership.
Asia Pacific Skin Replacement Market Analysis and Trends
Meanwhile, Asia Pacific exhibits the fastest growth, with a CAGR surpassing 12% fueled by expanding medical infrastructure in China, India, and Southeast Asia. Government initiatives focusing on wound care and rising incidence of burn injuries accelerate market penetration. Market players have ramped up investment and localized manufacturing to tap into this burgeoning opportunity
Skin Replacement Market Outlook for Key Countries
USA Skin Replacement Market Analysis and Trends
The USA remains the largest market globally, driven by significant healthcare expenditure and widespread adoption of innovative treatment modalities. Recent data showed that in 2024, over 1.1 million procedures involving skin substitutes were performed across major hospitals, up 7% from the previous year. Industry players like Smith & Nephew and Organogenesis continue to expand their product lines to meet sophisticated demand dynamics, leveraging advanced clinical trials with outcomes supporting faster wound closure. Regulatory relaxations around regenerative medicine and increased reimbursement for skin substitutes under Medicare provide additional market momentum.
India Skin Replacement Market Analysis and Trends
India's market is rapidly evolving due to rising diabetic populations and an increasing number of trauma cases. The government’s push to modernize burn care centers and promote indigenous manufacturing has stimulated local demand. In 2024, imports of biologic skin substitutes grew 15% year-over-year, while domestic companies like L&C BIO expanded capacity to cater to cost-sensitive markets. Affordable healthcare initiatives and improved access to specialty clinics foster business growth, establishing India as a strategic future market.
Analyst Opinion
An increasing number of hospital admissions related to chronic wounds and burns has significantly expanded the demand for skin replacement solutions. For example, in 2024, hospital admissions due to diabetic foot ulcers alone increased by 6.8% globally compared to the previous year, pushing the market toward higher consumption of advanced skin substitutes.
Pricing dynamics heavily influence the market growth trajectory; premium-priced bioengineered skin products captured over 42% share of the total skin replacement market revenue in 2024, driven by superior clinical efficacy despite affordability challenges in emerging economies.
Import statistics emphasize rising cross-border trade in skin replacement materials, with Asia Pacific's imports surging 15% year-over-year in 2024, underscoring the demand in fast-expanding healthcare infrastructure markets such as India and China.
Diverse use cases beyond wound healing, including cosmetic and reconstructive surgeries, are broadening end-user segments. Reports from leading burn treatment centers in 2025 highlight that 28% of skin substitutes’ application is now related to elective surgical repair, contributing to market size expansion.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 5.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 10.2% | 2033 Value Projection: | USD 11.3 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Allergan plc, TELA Bio, Inc., Acelity L.P. Inc., L&C BIO, Cytograft Tissue Engineering, Inc., Stratatech Corporation, BioLife Solutions, Inc., Stryker Corporation, Hans Biomed Corporation, Medtronic plc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Skin Replacement Market Growth Factors
The rising global prevalence of chronic wounds, projected to exceed 8 million cases annually by 2027, is a critical market driver. Increasing incidence is linked directly to aging populations and diabetes prevalence. Additionally, continuous advancements in bioengineering technologies have enabled development of more effective and safer skin substitutes, increasing user acceptance and willingness to pay premium prices. Another prominent driver is expanding reimbursement frameworks in developed economies, where updated policies in 2024 improved coverage for advanced skin replacement therapies. Furthermore, accelerating demand from emerging markets driven by growing healthcare infrastructure investments and rising patient awareness is shaping the market dynamics positively.
Skin Replacement Market Development
In April 2025, AVITA Medical launched Cohealyx, a next-generation collagen matrix designed to significantly reduce the preparation time for graft readiness. The product supports faster wound bed integration and is aimed at improving efficiency and outcomes in complex wound and burn management, particularly where rapid graft availability is critical.
In April 2025, LifeNet Health unveiled Dermacell Porous, an advanced acellular dermal matrix developed for complex and chronic wound applications. The product entered Medicare coverage under newly updated reimbursement guidelines, improving access for patients requiring advanced wound care solutions and strengthening LifeNet Health’s position in the regenerative medicine space.
In January 2025, Stanford Medicine received U.S. FDA approval for genetically engineered skin grafts targeting patients with epidermolysis bullosa. Clinical results demonstrated an 81% healing rate, marking a major breakthrough for this rare genetic condition and highlighting the growing role of gene-engineered regenerative therapies in severe dermatological disorders.
Key Players
Leading Companies of the Market
Allergan plc
TELA Bio, Inc.
Acelity L.P. Inc.
L&C BIO
Cytograft Tissue Engineering, Inc.
Stratatech Corporation
BioLife Solutions, Inc.
Stryker Corporation
Hans Biomed Corporation
Medtronic plc
Several market players have adopted strategic partnerships to expand their product portfolios and geographic reach. For instance, a notable collaboration between CollPlant Biotechnologies and a leading regenerative medicine company in 2024 helped reinforce CollPlant’s market penetration in Asia Pacific, contributing to a 9% increase in its regional revenue. Additionally, Smith & Nephew's acquisition of a synthetic skin substitute firm in 2025 augmented its competitive positioning by enabling bundled wound care solutions with documented clinical benefits.
Skin Replacement Market Future Outlook
The future of the skin replacement market is expected to be shaped by regenerative medicine and advanced biomaterials. Innovations such as stem cell-based constructs and 3D-bioprinted skin will enhance functional and aesthetic outcomes. Demand will rise with increasing incidence of burns, chronic wounds, and aging populations. Personalized skin replacements tailored to patient-specific needs may become more prevalent. Ongoing clinical research and technological advancements will drive long-term market expansion.
Skin Replacement Market Historical Analysis
The skin replacement market evolved from early skin grafting techniques used in burn and trauma care. Initially, autografts and allografts were the primary treatment options, limited by donor availability and rejection risks. Advances in tissue engineering led to the development of bioengineered skin substitutes incorporating synthetic and biological materials. These innovations improved healing outcomes and reduced infection rates. Clinical adoption expanded to chronic wounds, diabetic ulcers, and reconstructive surgeries. Regulatory approvals and reimbursement frameworks supported broader market penetration over time.
Sources
Primary Research Interviews:
Plastic Surgeons
Burn Care Specialists
Wound Care Nurses
Biomedical Engineers
Hospital Administrators
Databases:
NIH Tissue Engineering Data
PubMed
MedTech Europe
Magazines:
Wound Care Advisor
Medical Design & Outsourcing
MedTech Insight
Healthcare Innovation
BioSpectrum
Journals:
Burns Journal
Journal of Tissue Engineering
Wound Repair and Regeneration
Biomaterials
Regenerative Medicine
Newspapers:
The Guardian (Health)
New York Times (Health)
Financial Times (Healthcare)
Reuters Health
The Hindu (Health)
Associations:
American Burn Association
International Society for Burn Injuries
Tissue Engineering and Regenerative Medicine International Society
Wound Healing Society
European Burns Association
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients