The skin barrier products market is estimated to be valued at USD 1,594.1 Mn in 2025 and is expected to reach USD 2,228.1 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 4.9% from 2025 to 2032. Skin barrier films are liquid formulations that protect the skin from mechanical or chemical injury and are used in post ostomy/stoma surgery. Skin barrier films are also used in the prevention of moisture-associated skin damage (MASD) which is excessive moisture caused by incontinence, perspiration, or wound drainage.
Products that form a transparent protective coating on the skin and can be safely applied to intact or broken skin without stinging may be made from a variety of substances such as acrylates, polymers both organic and inorganic, and silicone. Following the application, the barrier's liquid component evaporates, leaving a transparent, breathable protective coating.
Skin barrier films come in a variety of packaging and dispensing options such as foam applicators, sprays, wipes, and for the stoma, they come in tapes and strips.
Global Skin Barrier Products Market - Impact of the Coronavirus (COVID-19) Pandemic
The coronavirus (COVID-19) outbreak was first reported on December 31, 2019, in Wuhan, China. The World Health Organization declared the COVID-19 a pandemic on March 11, 2020. According to the Coronavirus (COVID-19) Weekly Epidemiological Update by the World Health Organization, over 399 million cases and 5.75 million deaths due to coronavirus disease (COVID-19) were reported up till February 9, 2025, across the globe.
Impact of COVID-19 on Demand and Supply of Skin Barrier Products
The COVID-19 pandemic and consequent lockdown in various countries across the globe have impacted the financial status of businesses across all sectors including the private healthcare sector. The COVID-19 pandemic has impacted the entire supply chain of the healthcare industry mainly due to strict lockdown in several regions. The COVID-19 pandemic has affected the economy of various regions across the globe in three main ways; 1) by directly affecting the production and demand; 2) by creating disruptions in distribution channels; 3) through its financial impact on companies and financial markets. Many countries such as Thailand, Indonesia, and Singapore are facing problems with regard to the transportation and distribution of healthcare products.
The COVID-19 caused significant disruption to the delivery of patient care across the globe, including ostomy management or stoma care. For instance, according to an article published in the British Journal of Nursing in September 2020, the number of face-to-face appointments and home visits of patients with a stoma and an ileo-anal pouch at Oxford University Hospitals NHS Foundation Trust decreased significantly between April and June 2020, causing difficulties for patients in ostomy management.
Figure 1: Global Skin Barrier Products Market Share (%) Analysis, By Product Type
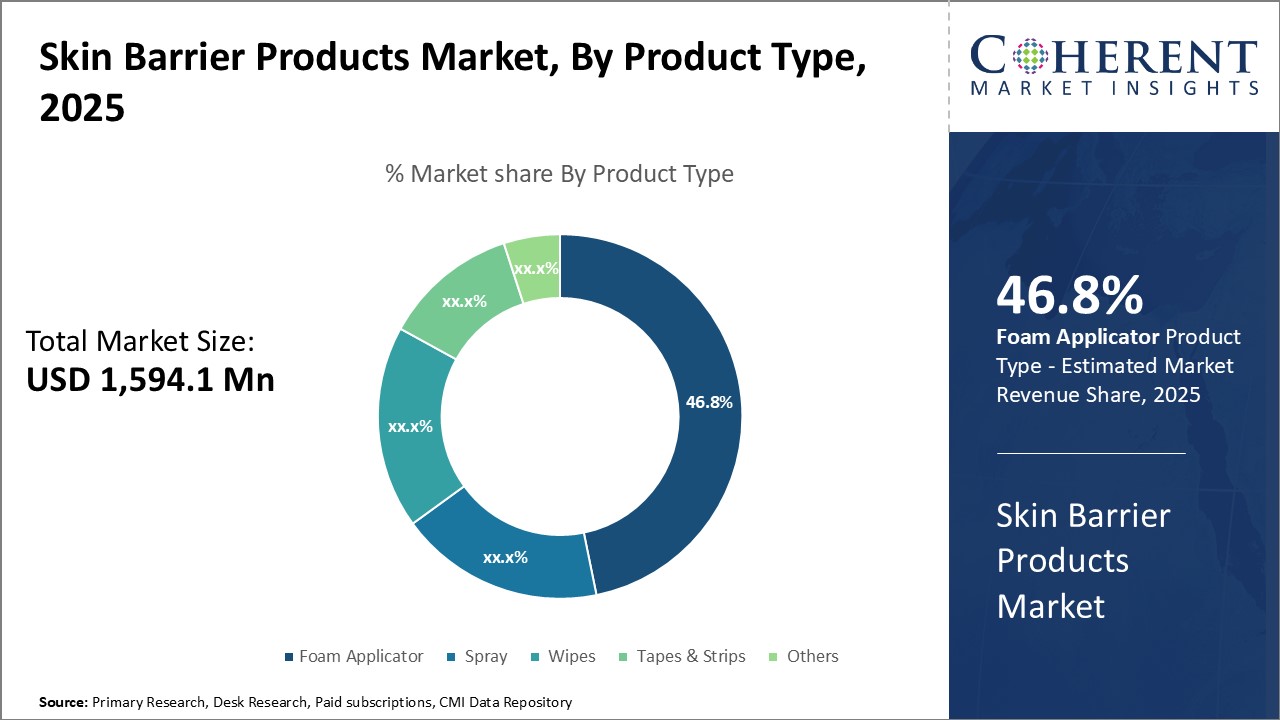
To learn more about this report, Download Free Sample
The increasing cases of incontinence is the major factor that is expected to drive the global skin barrier products market growth over the forecast period.
The emerging prevalence of urinary or fecal incontinence is expected to drive the growth of the global skin barrier products market. For instance, according to the Journal of Urology, in clinical setting, urinary incontinence is frequently underreported and underdiagnosed. The prevalence of urinary incontinence in the U.S. was 53% from 2005 to 2016; 16% of women had mixed urinary incontinence, 26% had stress only, and 10% had urgency only. While urgency and mixed urinary incontinence were most common in women aged over 60 years, stress urinary incontinence was most common in women aged 40–59 years.
Skin Barrier Products Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1,594.1 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.9% | 2032 Value Projection: | USD 2,228.1 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
3M, ConvaTec Inc., Coloplast Corp., Salts Healthcare, MEDLINE, Medicareplus International, Essity Medical Solutions, Cardinal Health, Smith & Nephew Plc., Safe n Simple, B Braun Medical Inc., Hollister Inc., and DermaRite Industries, LLC. |
||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Research and Development Activities
The increasing number of research and development activities by key market players is expected to aid in the growth of the global skin barrier products market over the forecast period. For instance, in September 2018, a controlled, randomized, prospective, open-label study was conducted in 21 healthy human volunteers to determine the durability of Cavilon Advanced Skin Protectant developed by 3M, an American multinational corporation, when applied to intact skin and compare it to three other products used for similar clinical indications. The 3M skin protectant was found to be more durable than the other products tested. It stayed on the skin for up to 7 days for all participants, whereas the other products had only 50% left on the skin at that point.
Global Skin Barrier Products Market – Regional Analysis
On the basis of region, the global skin barrier products market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa.
North America is expected to hold a dominant position in the global skin barrier products market over the forecast period, owing to new product launches of skin barrier wipes by key market players. For instance, in March 2017, ConvaTec Group Plc., a leading global medical technology company, announced the launch of Sensi-Care Skin Protectant Incontinence Wipes in the U. S., offering customers an advanced solution to help protect patients whose skin is at the risk of breakdown due to incontinence.
Moreover, due to increasing acquisitions by key market players, Europe is expected to witness significant growth in the global skin barrier products market over the forecast period. For instance, in January 2017, ConvaTec Group Plc, a leading global medical products and technologies company, announced the acquisition of EuroTec Beheer B.V., a Dutch-based manufacturer of ostomy appliances, for US$ 28 Million. Skin barrier products are used as ostomy pouch accessories. Furthermore, the addition of EuroTec to the ConvaTec family significantly strengthened their ostomy care business in France and the Benelux union.
Figure 2: Global Skin Barrier Products Market Value (US$ Mn), By Region
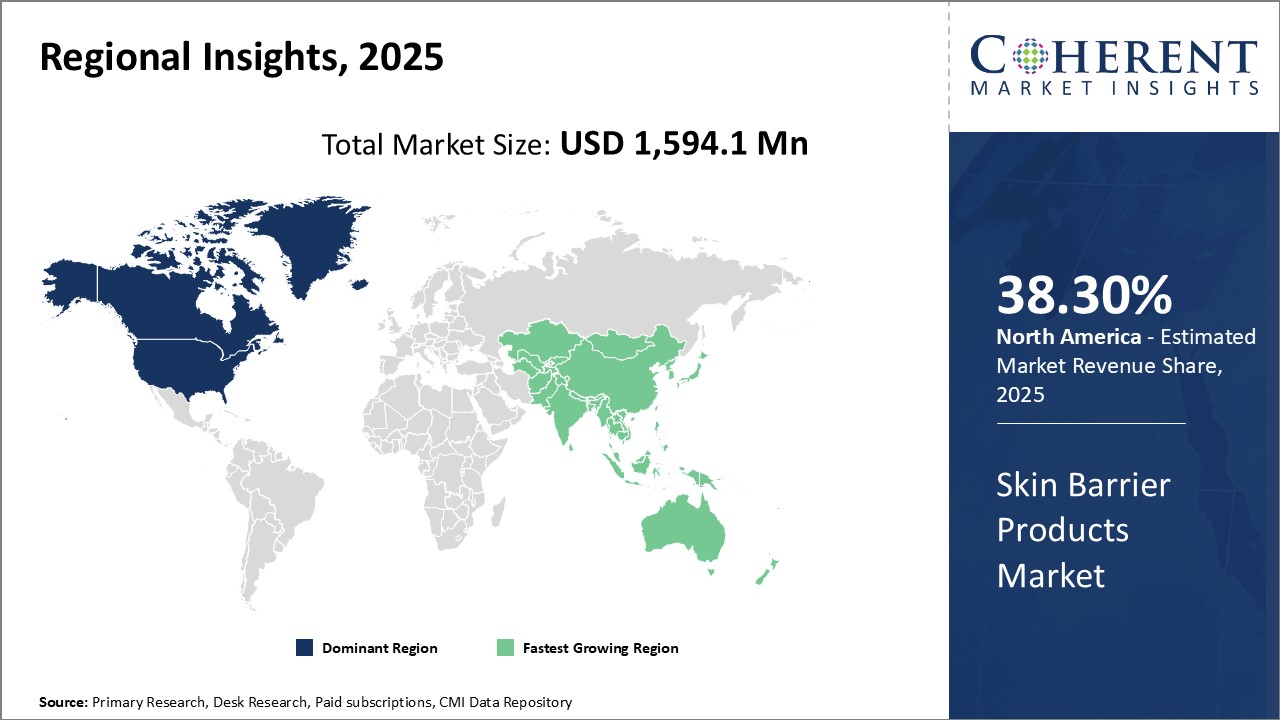
To learn more about this report, Download Free Sample
Key Developments:
*Definition: Skin barrier films are liquid formulations that protect the skin from mechanical or chemical injury and are used in post ostomy/stoma surgery. Skin barrier films are also used in the prevention of moisture-associated skin damage (MASD) which is excessive moisture caused by incontinence, perspiration, or wound drainage. Products that form a transparent protective coating on the skin and can be safely applied to intact or broken skin without stinging may be made from a variety of substances such as acrylates, polymers both organic and inorganic, and silicone. Following the application, the barrier's liquid component evaporates, leaving a transparent, breathable protective coating. Skin barrier films come in a variety of packaging and dispensing options such as foam applicators, sprays, wipes, and for the stoma, they come in tapes and strips.
Global Skin Barrier Products Market – Competitive Landscape
Major players operating in the global skin barrier products market include 3M, ConvaTec Inc., Coloplast Corp., Salts Healthcare, MEDLINE, Medicareplus International, Essity Medical Solutions, Cardinal Health, Smith & Nephew Plc., Safe n Simple, B Braun Medical Inc., Hollister Inc., and DermaRite Industries, LLC.
Market Segmentation
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients