Sirolimus Market Size and Forecast – 2025 – 2032
The Global Sirolimus Market size is estimated to be valued at USD 1.25 billion in 2025 and is expected to reach USD 2.15 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 8.1% from 2025 to 2032.
Global Sirolimus Market Overview
Sirolimus (rapamycin) is an immunosuppressive and antiproliferative pharmaceutical drug primarily used to prevent organ transplant rejection and coat coronary stents to reduce restenosis. It works by inhibiting the mTOR pathway, regulating immune responses and cell growth, making it valuable in transplantation medicine and emerging oncology research.
Key Takeaways
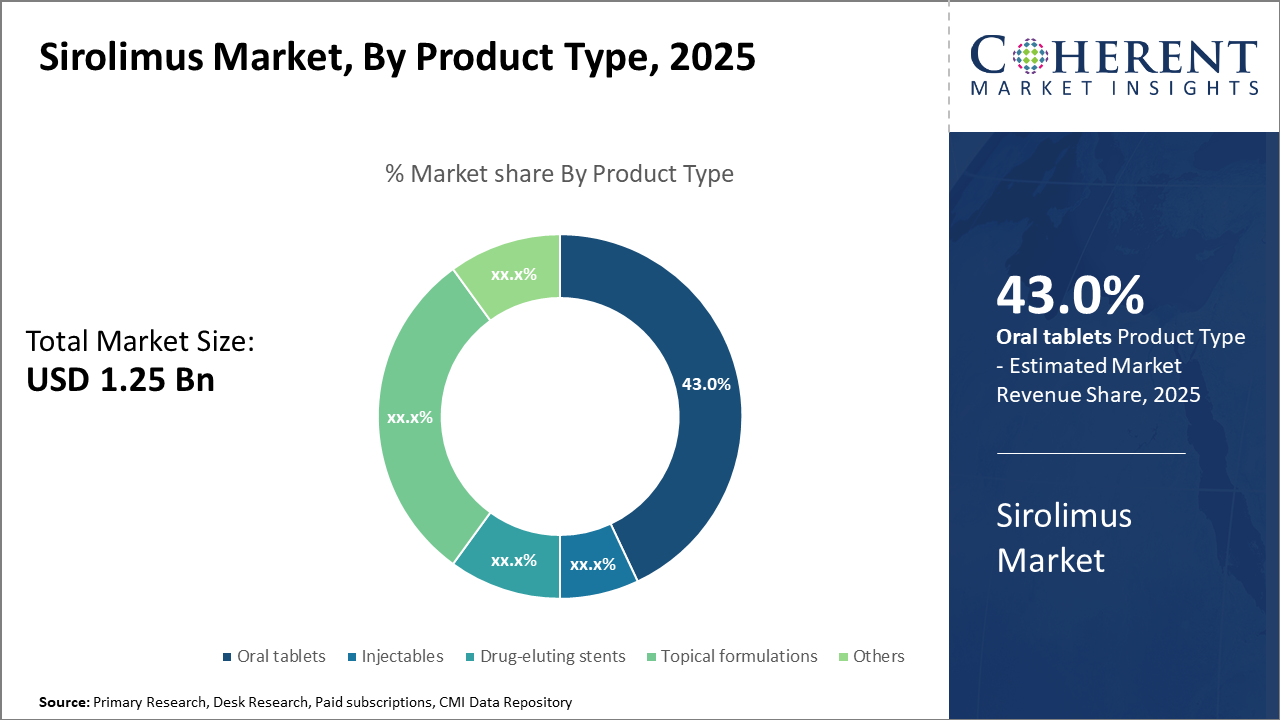
The oral tablets segment dominates the product type market due to convenience and patient compliance, accounting for 43% of industry share, driven by robust prescription volumes.
Hospitals remain the dominant end-user segment, capturing 56% market share because of concentrated transplant and cardiovascular care facilities, ensuring steady demand.
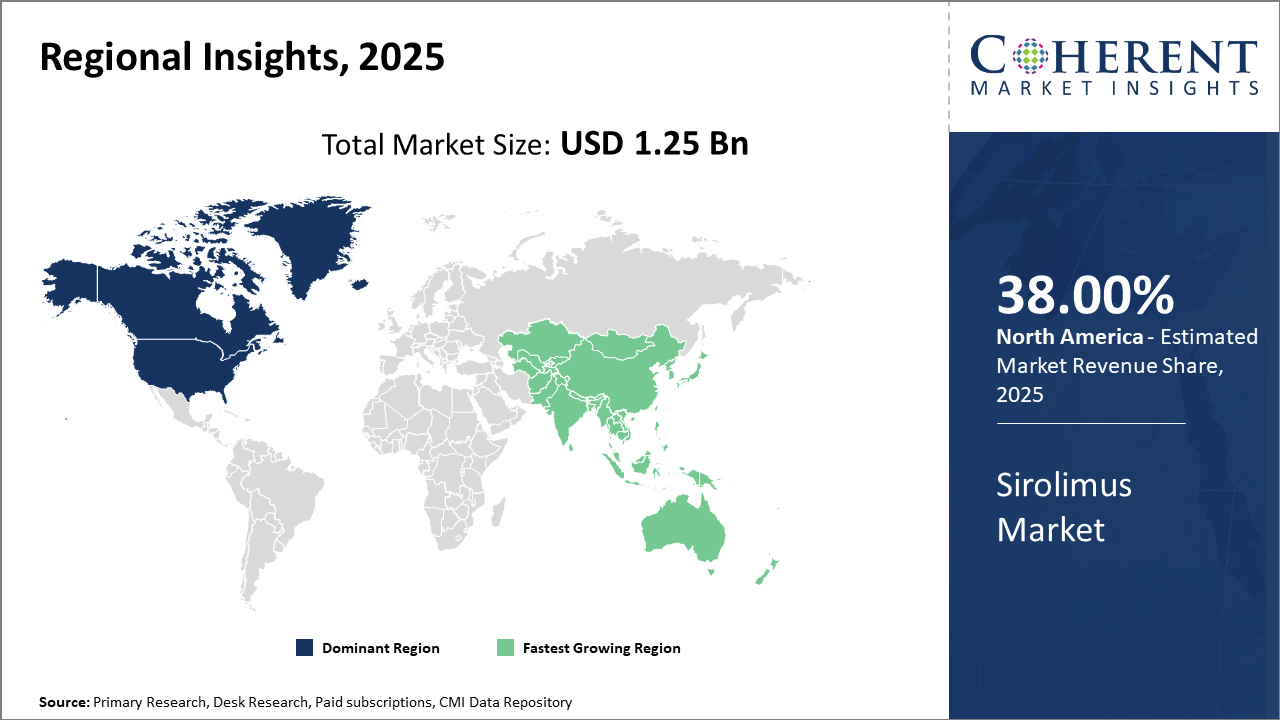
North America leads the regional market share, owing to advanced healthcare infrastructure and extensive organ transplant procedures, contributing over 38% to global industry revenue.
Asia Pacific exhibits the fastest market growt,h propelled by increasing organ transplant rates, government support, and expanding cardiovascular disease prevalenc,e with a CAGR surpassing 9%.
Sirolimus Market Segmentation Analysis
To learn more about this report, Download Free Sample
Sirolimus Market Insights, By Product Type
Oral tablets dominate the market share due to ease of administration, consistent dosing, and enhanced patient adherence, contributes significantly to industry revenue streams. The rising preference for fixed-dose combinations and sustained-release oral tablets also supports this segment’s expansion. Drug-eluting stents represent the fastest-growing subsegment, driven by increased cardiovascular interventions and newer stent approvals in multiple regions. Their application in preventing restenosis post-angioplasty drives demand in emerging markets. Injectables, primarily used during early post-transplant phases, retain steady growth supported by hospitals requiring controlled dosing environments.
Sirolimus Market Insights, By Application
Organ transplant immunosuppression dominates the market share, reflecting the long-standing primary use of sirolimus in preventing graft rejection, driven by increasing transplant surgeries globally. Oncology is the fastest-growing subsegment, buoyed by emerging clinical evidence supporting sirolimus’s role in inhibiting tumor growth pathways in cancers such as renal cell carcinoma and lymphoma. Cardiovascular applications, particularly drug-eluting stents, continue to expand rapidly as a complementary indication. Autoimmune disorders maintain a moderated growth owing to selective off-label use.
Sirolimus Market Insights, By End-User
Hospitals dominate the market share due to centralized transplant programs and specialized cardiovascular intervention units. The fastest-growing subsegment is Specialty clinics, particularly those focusing on autoimmune diseases and outpatient oncology, driven by expanding indications and enhanced treatment protocols involving sirolimus. Research institutes are pivotal in continuous clinical development, contributing to innovation, but are limited in commercial consumption. Ambulatory surgical centers and others have a minimal but growing presence as outpatient care models evolve with immunosuppressant treatments.
Sirolimus Market Trends
The Sirolimus market trend is characterized by accelerated adoption of biosimilar formulations since 2023, which has led to significant cost reductions, particularly in emerging economies such as India and Brazil.
For instance, generic sirolimus contributed to a 22% volume increase in these regions in 2024 by improving affordability.
Additionally, innovations in drug delivery, notably nanoparticle carriers developed by leading companies, have reported improved bioavailability by over 18% in clinical studies conducted in 2024, enhancing patient outcomes and encouraging broader prescribing across therapeutic segments.
Sirolimus Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Sirolimus Market Analysis and Trends
In North America, the dominance in the Sirolimus market stems from a well-established healthcare infrastructure, widespread organ transplant programs exceeding 40,000 annually, and strong R&D investments. Market revenue here accounts for over 38% of the global share. The U.S. leads with early adoption of advanced drug delivery technologies and biosimilars, supported by favorable policies promoting immunosuppressant usage.
Asia Pacific Sirolimus Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth, driven by increasing organ transplant volumes, growing cardiovascular disease prevalence, and government reimbursement initiatives. The region’s market CAGR surpasses 9%, accelerated by expanding healthcare access and aggressive pricing strategies by market players.
Sirolimus Market Outlook for Key Countries
USA Sirolimus Market Analysis and Trends
The USA's Sirolimus market benefits from a mature organ transplantation sector, with over 39,000 procedures performed in 2024 and a growing elderly population demanding targeted immunosuppressive therapy. Leading pharmaceutical companies have actively launched biosimilar versions and novel formulations, enhancing treatment accessibility and driving approximately 42% of North America's market revenue. Strategic partnerships and regulatory incentives for biosimilars further augment business growth in this region.
India Sirolimus Market Analysis and Trends
India's Sirolimus market is rapidly expanding due to increasing organ transplant surgeries, which surged by nearly 15% in 2024, paired with a rising incidence of autoimmune disorders. The presence of several generic manufacturers offering cost-effective sirolimus formulations has enhanced market penetration, particularly in tier-2 and tier-3 cities. Government initiatives supporting healthcare affordability also fostered a 12% growth rate in the sirolimus market revenues, positioning India as a vital growth engine in the Asia Pacific region.
Analyst Opinion
The rising prevalence of organ transplant procedures worldwide directly correlates with the increasing sirolimus market size. In 2024, over 136,000 organ transplants were performed globally, up 6% from the previous year, significantly boosting demand for immunosuppressive drugs like sirolimus. Market revenue is also positively impacted by expanding indications in renal and cardiac transplant patients, who increasingly prefer sirolimus-based regimens due to fewer nephrotoxic effects compared to alternatives.
Production capacity for advanced sirolimus formulations has expanded notably, with key pharmaceutical manufacturers scaling up bioprocessing capabilities. In 2025, manufacturing output capacity increased by approximately 12% worldwide, driven by investments in novel drug delivery systems aiming to improve bioavailability and reduce dosing frequency, thereby opening new market segments and enhancing treatment adherence.
On the demand side, the approval of sirolimus-eluting coronary stents in multiple regulated markets has considerably diversified the application spectrum. The Asia Pacific region, particularly South Korea and Japan, recorded more than 15,000 implantations in 2024, signifying a burgeoning use-case beyond transplantation. This market shift is expected to influence overall market growth positively and broaden industry share dynamics.
Price optimization strategies employed by market players, focusing on biosimilar and generic versions, have also affected market share distribution. In 2024, generics accounted for nearly 34% of the total market revenue, increasing accessibility, especially in emerging economies. This trend supports predicted increases in market scope, with biosimilars penetrating new geographical markets, reducing market restraints linked to high cost therapies.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: |
USD 1.25 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.1% | 2032 Value Projection: |
USD 2.15 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Pfizer Inc., Novartis AG, Johnson & Johnson, Sanofi S.A., Mylan N.V., Sandoz (part of Novartis), Teva Pharmaceutical Industries Ltd., Cipla Ltd., Dr. Reddy’s Laboratories, Lupin Limited. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Sirolimus Market Growth Factors
The market growth is primarily driven by the increasing number of organ transplant surgeries worldwide, coupled with the rising incidence of autoimmune diseases, where sirolimus’s immunosuppressive properties are vital. Technological advancements in drug delivery systems, including sustained-release and nanosuspension formulations, have improved patient compliance, thus driving increased adoption. Regulatory approvals for sirolimus in oncology and cardiovascular interventions, such as drug-eluting stent,s have opened new high-growth avenues. Additionally, growing investment in biosimilar development by pharmaceutical companies is expected to lower therapy costs and broaden accessibility, stimulating market expansion in emerging markets.
Sirolimus Market Development
In April 2022, the topical sirolimus gel Hyftor received U.S. FDA approval for the treatment of facial angiofibroma associated with tuberous sclerosis complex (TSC) in both adults and pediatric patients. The approval marked the first topical mTOR inhibitor available for this indication, offering a non-invasive therapeutic option with targeted dermatologic delivery.
In November 2021, the injectable sirolimus protein-bound formulation Fyarro was approved by the U.S. FDA for adults with advanced perivascular epithelioid cell tumor (PEComa). This represented the first FDA-approved therapy specifically for advanced PEComa and utilizes albumin-bound nanoparticle technology to improve drug delivery and tumor uptake.
Key Players
Leading Companies of the Market
Pfizer Inc.
Novartis AG
Johnson & Johnson
Sanofi S.A.
Mylan N.V.
Sandoz (part of Novartis)
Teva Pharmaceutical Industries Ltd.
Cipla Ltd.
Lupin Limited
Major market companies have adopted several aggressive growth strategies, including strategic partnerships and regional expansions in emerging markets. For example, Pfizer entered a licensing agreement in 2024 with a leading Asian pharmaceutical entity to co-develop sirolimus generics, resulting in a 15% increase in market share in that region within nine months. Additionally, mergers and acquisitions have helped market players consolidate their production footprint, such as Johnson & Johnson acquiring a biopharma company specializing in sirolimus delivery technologies, which enhanced their product portfolio and led to a 12% revenue increase in 2024.
Sirolimus Market Future Outlook
The market outlook is increasingly shaped by innovation in delivery mechanisms—such as topical, extended-release, inhaled, and nanoparticle formulations—that aim to improve tolerability and site-specific therapeutic response. Rising transplantation rates globally, coupled with growing demand for cost-effective immunosuppressive therapy, will reinforce long-term market stability. Meanwhile, ongoing clinical trials investigating sirolimus in cancer, autoimmune disorders, and vascular anomalies may unlock new revenue pathways. Expanding healthcare infrastructure in emerging markets, supportive regulatory approvals, and precision medicine adoption are expected to further enhance growth prospects.
Sirolimus Market Historical Analysis
Historically, sirolimus established its commercial foundation as a breakthrough immunosuppressant for organ transplant maintenance therapy, significantly improving long-term graft survival. Its antiproliferative mechanism later positioned it as a key therapeutic in drug-eluting coronary stents, reshaping interventional cardiology by reducing restenosis rates. Patent expirations led to generic market entry, broadening treatment accessibility and accelerating adoption worldwide. Academic research also expanded interest in sirolimus for rare diseases, dermatologic conditions, and oncology, strengthening clinical relevance beyond transplant medicine.
Sources
Primary Research Interviews:
Transplant Physicians
Hospital Pharmacists
Biopharma Executives
Databases:
ClinicalTrials.gov
IQVIA Drug Sales Data
FDA Drug Approval Database
Magazines:
PharmaTimes
BioPharma Dive
Pharmaceutical Technology
Journals:
Lancet Oncology
American Journal of Transplantation
Journal of Controlled Release
Newspapers:
The Washington Post (Health)
The Times of India (Healthcare)
Reuters (Pharma)
Associations:
International Society for Heart and Lung Transplantation (ISHLT)
American Society of Transplantation (AST)
EMA
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients