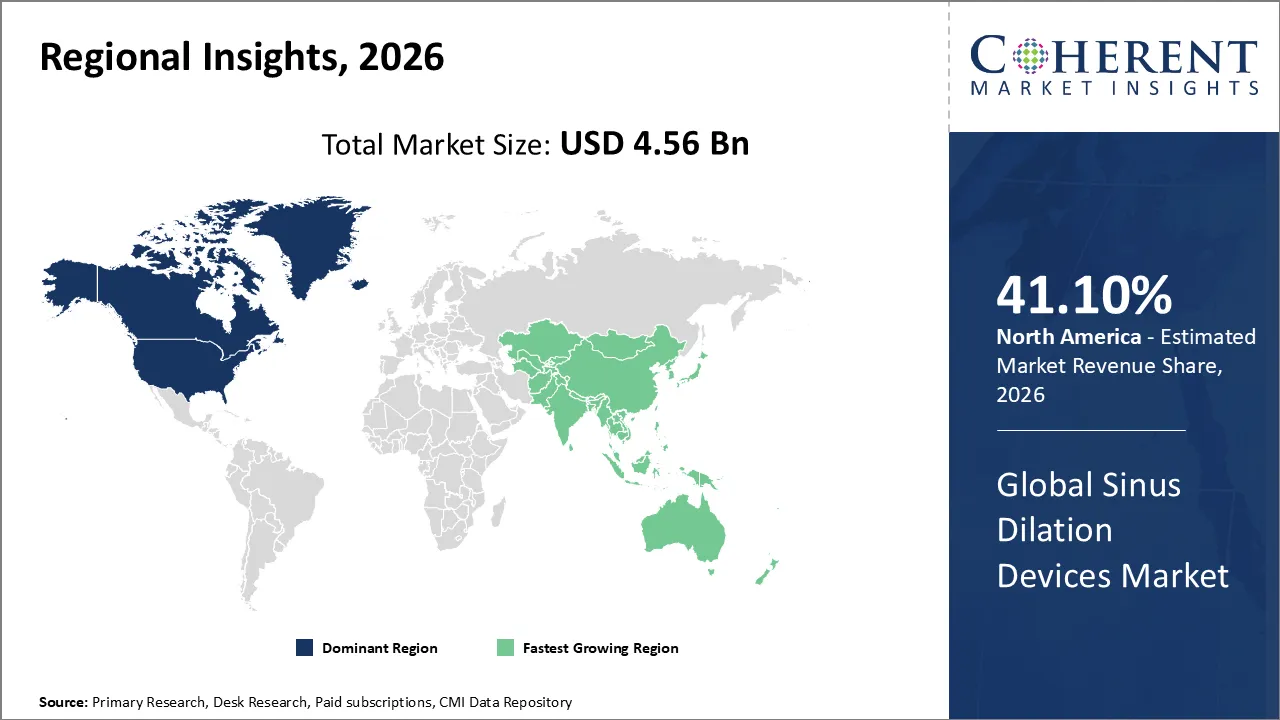
The Sinus Dilation Devices Market is estimated to be valued at USD 4.56 Bn in 2026 and is expected to reach USD 7.89 Bn by 2033, exhibiting a compound annual growth rate (CAGR) of 11.2% from 2026 to 2033.
The market for sinus dilation devices is expanding rapidly as chronic sinusitis becomes more common and doctors are more prepared to employ less intrusive treatments. More and more hospitals, clinics, and outpatient facilities are using equipment like balloon sinuplasty devices to help patients heal faster, with fewer complications, and feel better. More consumers are adopting these items as a result of payment limits and increased awareness of health concerns. Manufacturers are also developing new concepts employing cutting-edge technology. North America has the largest market share because to its contemporary infrastructure, trained ENT specialists, and a large population that wants the latest sinus treatments.
|
Current Events |
Description and its impacts |
|
Geopolitical and Regulatory Developments |
|
|
Technological Advancements and Innovation |
|
|
Epidemiological and Population Health Trends |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Balloon Sinuplasty Systems will hold the largest market share of 43.2% in 2026. Balloon sinuplasty devices are becoming increasingly popular in the sinus dilatation device sector, as clinicians favor procedures that preserve natural sinus structure. These therapies are typically administered under local anesthesia by physicians in office settings, reducing reliance on hospitals and overall care load. Positive clinical outcomes and long-term symptom relief increase physician confidence and promote widespread adoption. At the same time, ongoing advances in device design, usability, and procedural efficiency remain balloon sinuplasty a popular choice among patients and ENT practitioners.
For instance, Acclarent, Inc. recently launched the RELIEVA SpinPlus Balloon Sinuplasty System in the United States, featuring integrated irrigation that allows lavage of the targeted sinus.
By 2026, hospitals and clinics are predicted to hold the most share of the market (54.4%). As the number of sinus disease cases rises, hospitals and clinics are needing more sinus dilation devices since they are offering more minimally invasive ENT treatments. They conduct surgeries safely and successfully because to their cutting-edge diagnostic technologies, excellent surgical infrastructure, and highly qualified staff. These facilities make sinus dilatation a routine element of therapeutic operations, which helps more patients get therapy and makes it more successful. Hospitals and clinics also use this technology to give patients the care they need in well-regulated medical settings, with a lower chance of problems and a speedier recovery.
To learn more about this report, Download Free Sample
According to predictions, North America will have 41.10% of the world market in 2026. There are changes in the market for sinus opening devices in North America as more doctors use minimally invasive therapies and ENT treatments in the office. A lot of complex imaging and guidance tools help doctors do their jobs better and keep their patients safe. Focusing more on outpatient care, educating patients more, and finding sinus diseases early all make it more likely that acceptance will happen over time. Also, hospitals, clinics, and specialized ENT facilities are changing how they treat patients because new products are coming out all the time and doctors are getting better training.
For instance, Medtronic plc launched the FDA-cleared NuVent™ Eustachian tube dilation balloon for treating chronic obstructive Eustachian tube dysfunction.
There are more people getting modern ENT treatments in developing countries, which is making the Asia-Pacific sinus expansion device market grow. More people are getting nose infections because towns are getting bigger and more pollution is getting into the air. This is making more people want treatments that work. As more doctors and patients learn about less invasive ways to treat nose problems, more hospitals and specialty clinics begin to carry them out. Regional usage trends are also affected by the rise of medical tourism, efforts by the government to improve healthcare, and more money from global device makers.
In the US, the market for sinus dilation devices is rising quickly because doctors are using patient-centered, minimally invasive ENT procedures that are backed by new clinical practices. More and more, doctors are doing sinus dilation in the office and on an outpatient basis to make treatment easier and faster. To make procedures faster and safer, doctors use computerized images, navigation systems, and one-time use gadgets. Sinus dilation technology is also used in many healthcare settings because of strong clinical study, new products coming out all the time, and insurance plans that cover a lot of it.
The market for sinus dilation devices in China is growing because hospitals and ENT clinics are using less invasive ways to treat more sinus-related illnesses. As patients and doctors learn more, there is a greater need for new treatments that help people get better and heal faster. By putting money into modern medical facilities and government-funded healthcare programs, hospitals and other healthcare providers are speeding up the adoption of technology. At the same time, foreign companies are entering the market with new sinus dilation technologies that are changing how healthcare centers in both big cities and smaller towns do their work.
Minimally invasive sinus dilation treatments, such as balloon sinuplasty, are becoming more popular among healthcare providers than traditional surgeries. These techniques minimize tissue stress, reduce complication risks, and shorten recovery times. Hospitals, clinics, and office-based facilities are incorporating these devices into everyday ENT practices to meet patient demand for faster, safer treatments. This trend is also driven by procedural efficiency, increased physician confidence, and the capacity to undertake interventions in outpatient settings using local anesthetic.
The market is seeing a strong push to combine sinus dilation devices with improved imaging and navigation technologies. Real-time visualization improves procedural precision, particularly in complex sinus anatomy, and lowers the risk of complications. Providers are implementing systems with integrated imaging support and computer-assisted navigation to increase procedural safety, accuracy, and overall clinical results. This trend emphasizes the growing importance of technology-based solutions in ENT treatment.
Continuous R&D in sinus dilation technologies enables manufacturers to offer next-generation devices. Product differentiation and physician adoption can be achieved through innovations such as integrated navigation, single-use balloons, flexible catheters, and better visualization tools. Companies that address procedural efficiency, patient comfort, and safety can position themselves as leaders, extend their product portfolios, and meet changing demands in both hospital and office-based ENT settings.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 4.56 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 11.2% | 2033 Value Projection: | USD 7.89 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Entellus Medical, Inc., Smith & Nephew Plc., Acclarent, Inc., B. Braun Melsungen AG, Medtronic Plc, Olympus Corporation, SinuSys Corporation, InnAccel Technologies, and Intersect ENT, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients