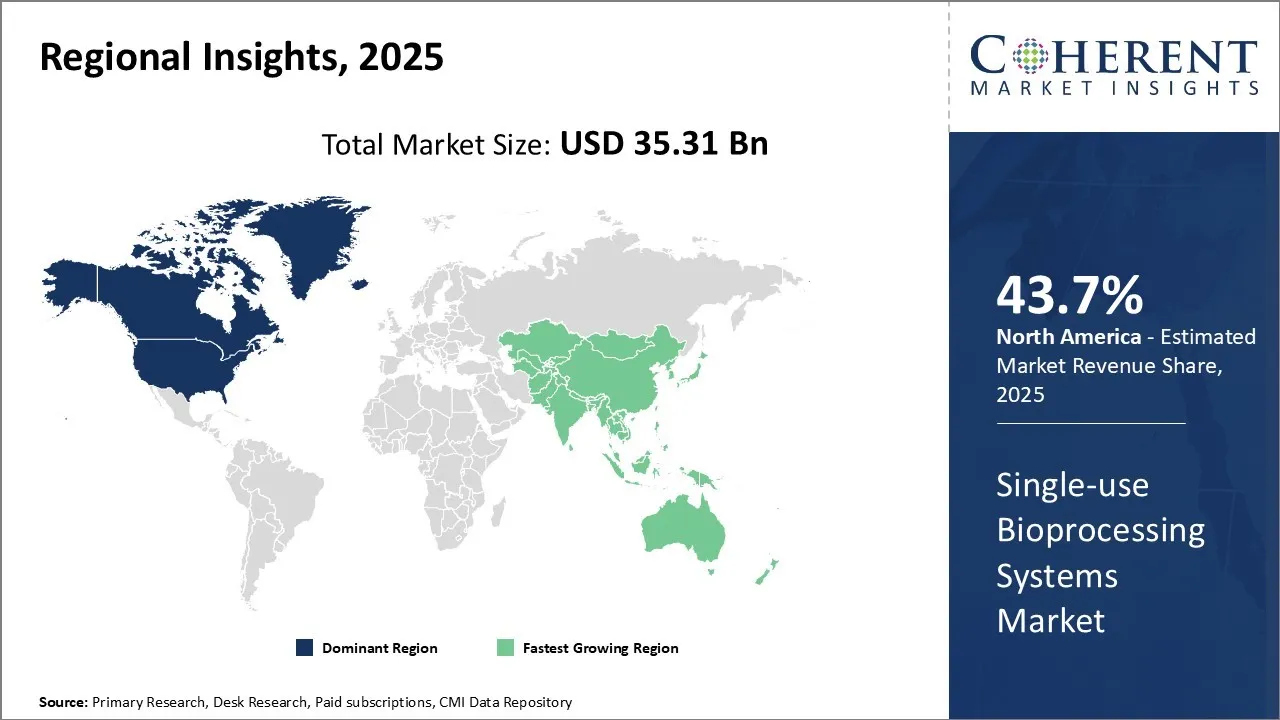
The global single-use bioprocessing systems market was valued USD 35.31 Bn in 2025 and is expected to reach USD 105.97 Bn by 2032 exhibiting a compound annual growth rate (CAGR) of 17% from 2025 to 2032.
The global single-use bioprocessing systems market is expected to grow significantly in the forecast period due to rising demand for low-cost, flexible, and contamination-free biomanufacturing options. The growth in the manufacture of biologics such as monoclonal antibodies, vaccines, and cell and gene therapies is driving adoption of disposable technologies in upstream and downstream processes.
Single-use systems offer key benefits, including lower cleaning needs, quicker batch turnaround, and improved sterility assurance, that make them especially appealing for clinical trials, contract manufacturing, and personalized medicine applications.
|
Current Events |
Description and its impact |
|
Biologics Manufacturing Growth Drives Increased Use of Single-use Bioprocessing Systems
|
|
|
Cell and Gene Therapy Surge Accelerates Bioprocessing Technologies in Single-use
|
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Single-use bioprocessing systems offer significant advantages in responding quickly to public health crises by enabling faster production timelines and flexible manufacturing capacities compared to traditional stainless-steel facilities. The pandemic response involved unprecedented investments and collaborations to develop and distribute vaccines in record time using single-use technologies.
Regulatory approvals are also faster since these systems present lower risks of cross-contamination. Going forward, regulatory agencies and governments are likely to emphasize the development of rapid vaccine production capabilities to prepare for future pandemics. Pfizer-BioNTech partnered with contract manufacturers like Thermo Fisher Scientific to deploy single-use technologies across multiple sites globally, facilitating flexible and rapid manufacturing scale-up.
The biopharmaceutical industry is witnessing rapid development and approval of complex novel biologic therapies such as monoclonal antibodies, gene therapies, and cell therapies to treat cancer, autoimmune diseases, and other serious medical conditions. Production of these complex biologics requires advanced manufacturing capabilities and highly controlled environments compared to traditional small molecule drugs.
Single-use bioprocessing systems are particularly well-suited for manufacturing such sensitive therapies since they eliminate issues like leachables, particles and bioburden associated with the reuse and cleaning of stainless-steel equipment.
As the pipeline of novel biologic drugs grows, especially those involving regenerative medicine and gene/cell therapy approaches, demand is increasing for specialized single-use technologies fitted with sensors and controllers that can carefully regulate critical manufacturing parameters.
The growing trend of outsourcing bioproduction processes to contract development and manufacturing organizations (CDMOs) has significantly impacted the global single-use bioprocessing systems market in recent years. More pharmaceutical and biotech companies are outsourcing their biomanufacturing needs to specialized CDMOs that offer flexible, cost-effective single-use technologies. This allows product developers to focus on their core competencies in R&D and reduces their capital investment in manufacturing infrastructure.
CDMOs are increasingly adopting standardized, pre-validated single-use platforms that offer speed, flexibility, and aseptic safety. They can easily customize the processes as per client requirements and handle fluctuating production demands. This has enabled even small biotech startups and mid-sized firms to access large-scale biomanufacturing capabilities.
Major elements of single-use bioprocessing infrastructure like bioreactors, mixers, tangential flow filtration devices, storage bags, and connectors are being rapidly added at several CDMO facilities. For example, Chinese CDMO WuXi Biologics has invested USD 600 million since 2018 to expand its aseptic drug substance manufacturing capacity based on standardized perfusion-based single-use bioprocessing technologies, as stated on their corporate website. This has propelled overall sales of single-use systems among leading suppliers.
Biologics have become the fastest-growing segment of the pharmaceutical market. This rapid growth of biologics drives the demand for single-use technologies that provide the benefits of flexible operations, easy scale-up, and lower initial capital investments.
Based on end user, Biopharmaceutical companies dominate the market. Biopharmaceutical companies dominate the single-use bioprocessing systems market due to their high demand for flexible, scalable, and contamination-free manufacturing processes.
These companies frequently develop biologics, which require stringent sterility and rapid production cycles—ideally suited for single-use technologies. Single-use systems reduce cleaning validation and downtime, enabling faster turnaround and lower operating costs.
To learn more about this report, Download Free Sample
The major market for single-use systems is the U.S., which is home to the majority of world's largest biopharma companies. These companies are actively investing in the development of novel biologics and biosimilars driving the need for single-use bioprocessing. Moreover, a supportive regulatory environment is also encouraging biopharma industries to incorporate single-use systems into their manufacturing operations to enhance flexibility and lower costs.
Local biopharma companies in Asia Pacific have ramped up investments to cater to growing healthcare demands as well as expand their international footprint. This has bolstered the sales of single-use bioprocessing equipment in the region.
The United States remains the leader in the single-use bioprocessing systems market due to a developed biopharmaceutical industry, high R&D spending, and high uptake of versatile manufacturing solutions. Thermo Fisher Scientific, Pall Corporation, and GE Healthcare are some of the big names based in the U.S., which bolsters innovation and local supply of cutting-edge single-use technologies. Growing emphasis on biologics and customized medicines also drives demand.
Germany dominates the European market with its robust biopharmaceutical production infrastructure and government backing of biotech innovation. Major CDMOs and biotech companies in the country depend strongly on single-use technology for upstream and downstream processes. A focus on GMP compliance and efficiency in production also hastens the adoption of disposable technology in bioprocessing.
China is becoming a force to be reckoned with in the single-use bioprocessing market, led by heavy government investment in biopharma facilities and a jump in home-grown biologics output. Domestic manufacturers are increasingly turning to single-use systems to minimize contamination risk and operating expenses. Collaborations with international suppliers such as Sartorius and Merck also are spurring adoption.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 35.31 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 17% | 2032 Value Projection: | USD 105.97 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Merck KGaA, Thermo Fisher Scientific Inc., Liquidyne, Corning Incorporated, Foxx Life Sciences., PARKER HANNIFIN CORP, Danaher Corporation., Sartorius, GE Healthcare, Eppendorf, Getinge AB, PBS Biotech, Esco Bioengineering, Distek, Bionet and Other Prominent Players |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients