Shingles Vaccine Market Size and Forecast – 2026 – 2033
The Global Shingles Vaccine Market size is estimated to be valued at USD 5.8 billion in 2026 and is expected to reach USD 11.6 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 11.2% from 2026 to 2033.
Global Shingles Vaccine Market Overview
The global shingles vaccine market is witnessing robust growth, driven by increasing awareness of shingles and its complications, particularly among the aging population. Vaccines like recombinant zoster vaccines are gaining prominence due to high efficacy and safety profiles. Rising healthcare expenditure, government immunization programs, and the growing prevalence of immunocompromised individuals further fuel market expansion. North America dominates the market, while Asia-Pacific shows significant growth potential. The market is also shaped by ongoing research, product innovations, and strategic collaborations among pharmaceutical companies. By 2033, the market is projected to nearly double, reflecting strong demand and adoption worldwide.
Key Takeaways
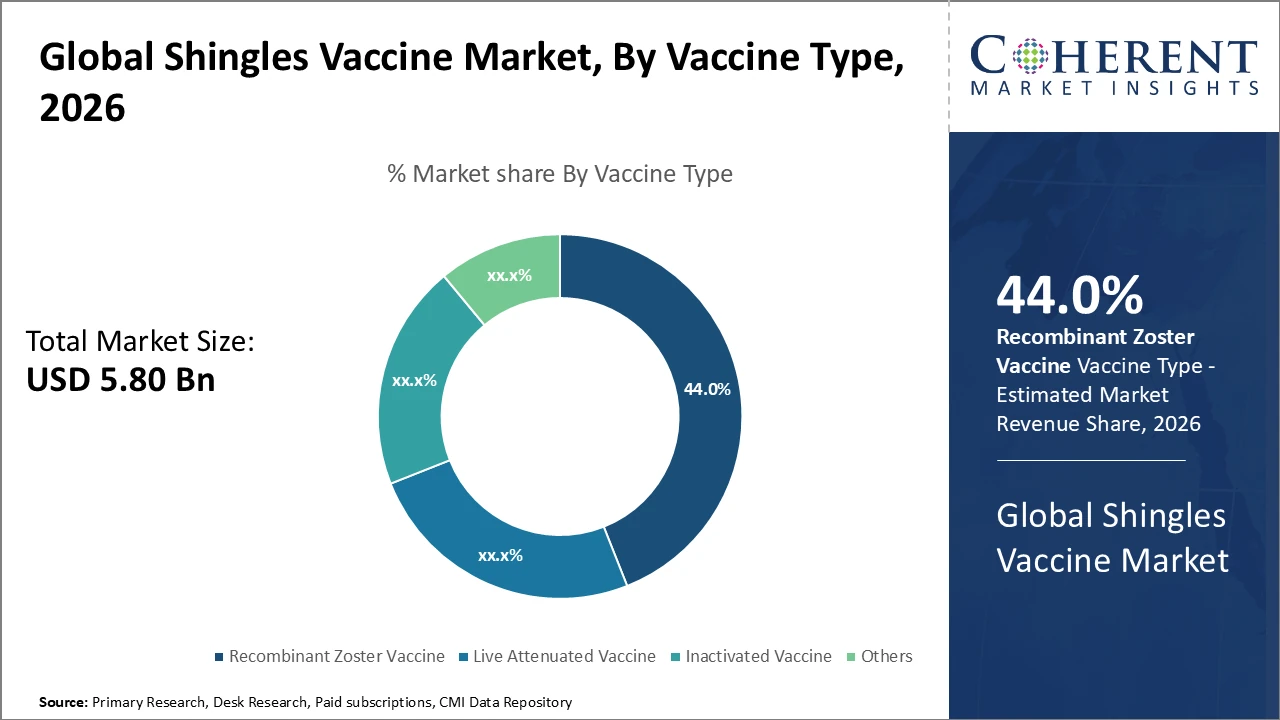
• Recombinant zoster vaccines dominate, capturing 44% market share in 2026 due to superior efficacy and expanded approvals.
• Hospitals lead the market because of their wide reach and integration within preventive care protocols; retail pharmacies are boosting growth by improving vaccine accessibility.
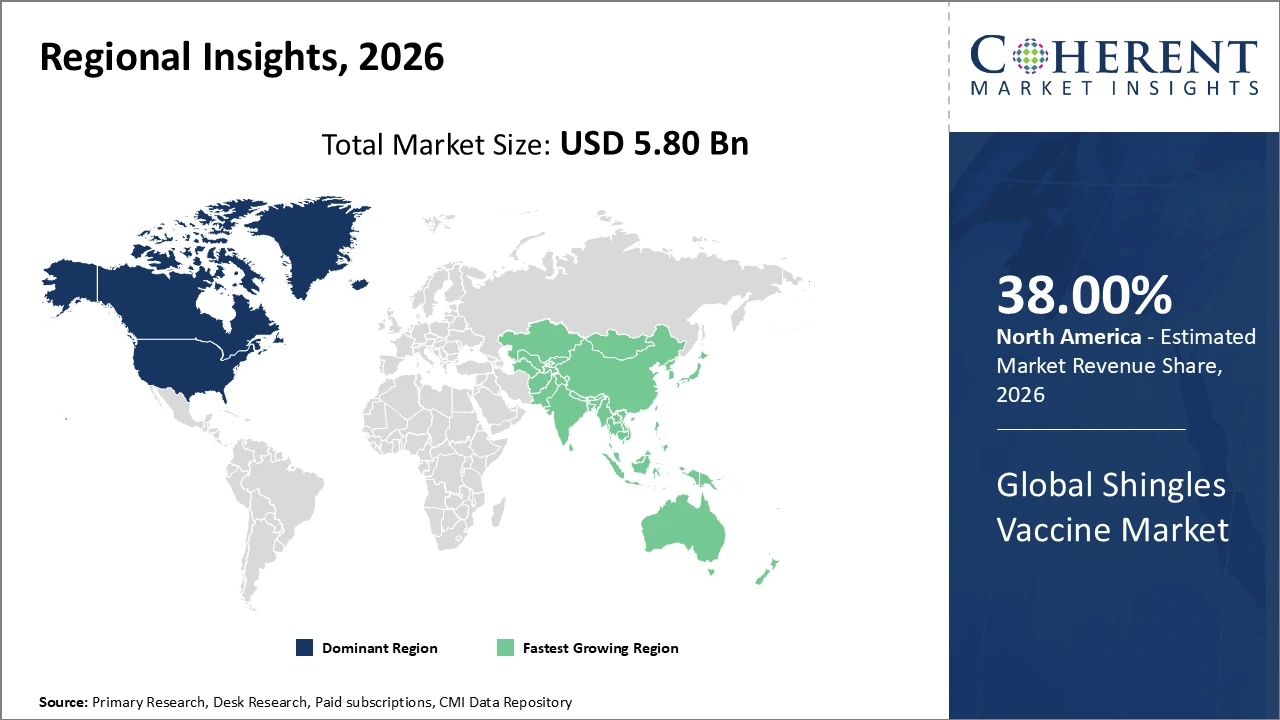
• North America holds the largest share, over 38% of revenue, driven by advanced healthcare infrastructure and comprehensive reimbursement programs.
• Asia Pacific exhibits the fastest CAGR, fueled by a growing geriatric population and government immunization initiatives, particularly in China and India.
Shingles Vaccine Market Segmentation Analysis
To learn more about this report, Download Free Sample
Shingles Vaccine Market Insights, By Vaccine Type
Recombinant Zoster Vaccine dominates the market with a 44% share, largely due to its enhanced safety profile and efficacy exceeding 90%, making it the preferred choice in immunization programs. Its rapid technological advancements and increasing production capacity are driving widespread adoption, especially in developed nations. Live-Attenuated Vaccines, while historically significant, face limitations in immunocompromised populations but continue to maintain niche demand in certain regions. Inactivated Vaccines are emerging, supported by ongoing clinical trials, offering promising growth potential. Other vaccine types, including experimental formulations, occupy smaller market segments with exploratory applications.
Shingles Vaccine Market Insights, By Distribution Channel
Hospital pharmacies hold the dominant market share due to their strategic position within healthcare facilities and direct access to patient populations. Retail pharmacies, while secondary, are growing rapidly thanks to increased vaccine availability, customer convenience, and endorsements from healthcare authorities. Online pharmacies are an emerging segment, showing notable growth as digital health platforms gain traction by providing vaccine information and facilitating telehealth appointments. The ‘Others’ category, including public health campaigns and non-traditional distribution models, contributes minimally but is experiencing gradual adoption driven by environmental and outreach initiatives. Hospital pharmacy channels remain crucial for sustaining high market penetration.
Shingles Vaccine Market Insights, By End-User
Hospitals serve as primary vaccination centers due to integrated adult immunization protocols and easy access to high-risk groups, ensuring efficient vaccine delivery and higher patient compliance. Clinics act as important community-level touchpoints, especially in rural and semi-urban areas, and are gaining market traction. Pharmacies are the fastest-growing segment, driven by rising consumer demand for convenient vaccination access and expanding pharmacy-led immunization services. The ‘Others’ category, including mobile clinics and workplace programs, contributes modest but growing adoption. While hospitals maintain steady demand through established infrastructure, pharmacies are fueling accelerated growth by leveraging broader consumer outreach.
Shingles Vaccine Market Trends
• Recent years have seen a strong shift toward recombinant vaccine adoption, driven by favorable efficacy and safety profiles demonstrated in 2024 and 2025 clinical studies.
• Digital health technologies supporting immunization tracking and patient engagement programs are enhancing vaccine adherence, especially among the elderly.
• There is a growing trend toward combination vaccines that integrate shingles with other adult immunizations, expected to reshape market revenue by offering comprehensive protection.
• Sustainability initiatives, including eco-friendly packaging and improved cold chain systems, are emerging in line with global healthcare policies on environmental stewardship.
Shingles Vaccine Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Shingles Vaccine Market Analysis and Trends
In North America, the shingles vaccine market is dominated by robust healthcare infrastructure, widespread immunization programs, and government reimbursements, including Medicare and Medicaid, which cover vaccination costs. The U.S. accounted for approximately 31% of global market revenue in 2026, supported by leading companies such as Pfizer and Merck driving continuous innovation and distribution. The region’s strong network of healthcare providers and integrated supply chains further reinforces its market position and resilience against global competition.
Asia Pacific Shingles Vaccine Market Analysis and Trends
Asia Pacific is experiencing the fastest growth in the shingles vaccine market, driven by a rising elderly population and strengthened government vaccination initiatives, particularly in China and India. The region’s CAGR exceeds 13% due to efforts to improve healthcare access and growing awareness of the vaccine’s benefits. Market players are increasingly focusing on collaborations and affordable vaccine options to capitalize on this emerging opportunity, with notable contributions from companies such as Serum Institute of India and Bharat Biotech.
Shingles Vaccine Market Outlook for Key Countries
USA Shingles Vaccine Market Analysis and Trends
The U.S. market holds a significant share of the shingles vaccine market, supported by extensive adult immunization programs and favorable reimbursement policies. In 2024, approximately 70% of eligible adults received the recombinant shingles vaccine, driven by awareness campaigns and healthcare provider recommendations. Leading companies such as Pfizer and Merck maintain dominance through ongoing R&D investments and expanded distribution networks. Both private and public healthcare sectors are contributing to higher vaccination uptake, aided by electronic health records and immunization registries that promote better compliance rates.
Germany Shingles Vaccine Market Analysis and Trends
Germany’s shingles vaccine market is experiencing steady growth, driven by an aging population and rising awareness of herpes zoster prevention. Recombinant vaccines, particularly Shingrix, dominate due to high efficacy and safety profiles. Government recommendations from STIKO and coverage through statutory health insurance enhance vaccine adoption among adults aged 50 and above. Hospitals, clinics, and pharmacies serve as key distribution channels, while digital health tools such as electronic reminders and telemedicine improve patient engagement and compliance. Despite high costs and occasional vaccine hesitancy, ongoing innovations, awareness campaigns, and collaborations among pharmaceutical companies are expected to sustain market expansion in the coming years.
Analyst Opinion
• The adoption of recombinant zoster vaccines over traditional live-attenuated vaccines has significantly driven market growth.
• In 2025, global production capacity for recombinant vaccines increased by nearly 25%, reflecting a focus on safer, more effective alternatives, supported by efficacy data showing a 90% reduction in shingles incidence.
• Increased immunization drives in North America and Europe, backed by government-funded programs, boosted market revenue by over 15% in 2024.
• Pricing strategies, including value-based agreements and revisions, enhanced vaccine accessibility in middle-income regions, leading to increased market share.
• Partnerships between healthcare providers and manufacturers, expanded cold chain logistics, and digital tracking technologies improved delivery efficiency by 20%, benefiting seniors and immunocompromised patients.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 5.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 11.2% | 2033 Value Projection: | USD 11.6 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | GlaxoSmithKline plc, Pfizer Inc., Merck & Co, Inc., Sanofi Pasteur, Moderna Inc., Johnson & Johnson, Novavax Inc., CSL Limited, Seqirus, Bharat Biotechh | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Shingles Vaccine Market Growth Factors
The expansion of immunization programs targeting adult and elderly populations is a key driver of market growth, reflected in the increasing inclusion of shingles vaccines in routine adult immunization schedules across multiple countries in 2025. Advances in vaccine formulation, particularly recombinant technologies, enhance efficacy and safety, making these vaccines the preferred choice and boosting market revenue. Strong government support through subsidy programs and reimbursement policies further amplifies uptake, as seen in the U.S., where Medicare coverage expansion led to a 14% rise in vaccination rates in 2024. Increased public awareness campaigns on herpes zoster risks and complications have also expanded market reach by improving consumer confidence and acceptance worldwide.
Shingles Vaccine Market Development
In January 2026, GSK received European Commission approval for Shingrix, its recombinant zoster vaccine, in a prefilled syringe format. This innovation eliminates the need for reconstitution, simplifying administration and improving convenience for healthcare providers. The approval supports broader rollout across EU countries, enhancing shingles prevention and strengthening adult immunization programs.
In October 2025, Dynavax presented data for its shingles vaccine candidate and launched a head-to-head clinical study against GSK’s Shingrix. The trial will compare efficacy, safety, and immune response, aiming to position Dynavax’s vaccine as a strong alternative. This development highlights growing competition and innovation in shingles prevention and adult immunization programs.
Key Players
Leading Companies of the Market
GlaxoSmithKline plc
Pfizer Inc.
Merck & Co., Inc.
Sanofi Pasteur
Moderna Inc.
Johnson & Johnson
Novavax, Inc.
CSL Limited
Seqirus
Bharat Biotech
Competitive strategies in the shingles vaccine market prominently focus on product innovation, with recombinant vaccine technology taking the lead. For instance, Pfizer’s development and FDA approval of its recombinant vaccine in 2024 expanded its market share by 18%, supported by clinical trial results demonstrating superior efficacy. Similarly, strategic acquisitions by Merck enhanced production capabilities and improved market penetration in emerging economies, resulting in a 12% growth in regional revenue. Sanofi’s collaboration with logistics partners strengthened cold chain distribution in the Asia Pacific region, contributing to a 10% increase in market revenue in 2025.
Shingles Vaccine Market Future Outlook
The shingles vaccine market is poised for strong growth over the coming decade, driven by rising awareness of herpes zoster risks, expanding geriatric populations, and broader inclusion of vaccines in adult immunization programs worldwide. Recombinant vaccines are expected to maintain dominance due to superior efficacy and safety, while innovations in combination vaccines and delivery technologies will further enhance adoption. Government support, reimbursement initiatives, and digital health tools promoting vaccine tracking and patient engagement will continue to boost coverage. Emerging markets, particularly in Asia Pacific, present significant opportunities, and ongoing R&D efforts promise next-generation vaccines, improved formulations, and wider global accessibility.
Shingles Vaccine Market Historical Analysis
The shingles vaccine market has evolved significantly since the introduction of the first live-attenuated vaccines in the early 2000s. Initially, adoption was slow due to limited awareness, moderate efficacy, and contraindications in immunocompromised populations. Market growth accelerated with the launch of recombinant zoster vaccines, which demonstrated over 90% efficacy and improved safety profiles, leading to widespread acceptance in North America and Europe. Over the past decade, expanding adult immunization programs, government-backed reimbursement policies, and aging populations have driven steady uptake. Advances in manufacturing, distribution, and awareness campaigns have progressively broadened market reach, setting the foundation for robust growth in recent years.
Sources
Primary Research Interviews:
Immunologists and Virologists
Infectious Disease Specialists
Healthcare Providers (Hospitals, Clinics, Pharmacies)
Vaccine Manufacturers and Distributors
Databases:
World Health Organization (WHO) Immunization Data
Centers for Disease Control and Prevention (CDC) Vaccine Statistics
OECD Health Data
Global Health Data Exchange (GHDx)
Magazines:
Vaccine News Daily
Pharmaceutical Technology
PharmaTimes
Immunization News
HealthTech Magazine
Journals:
Vaccine
Human Vaccines & Immunotherapeutics
Journal of Infectious Diseases
Clinical Vaccine Immunology
Journal of Immunology Research
Newspapers:
The New York Times (Health)
The Guardian (Health)
Financial Times (Healthcare)
The Hindu (Health)
Reuters Health
Associations:
World Health Organization (WHO)
Centers for Disease Control and Prevention (CDC)
International Society for Vaccines (ISV)
European Vaccine Manufacturers Association (EVM)
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients