Sexually Transmitted Diseases (STDs) Treatment Market Size and Forecast – 2025 – 2032
The Global Sexually Transmitted Diseases (STDs) Treatment Market size is estimated to be valued at USD 15.7 billion in 2025 and is expected to reach USD 25.4 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 7.3% from 2025 to 2032.
Global Sexually Transmitted Diseases (STDs) Treatment Market Overview
The STD treatment market comprises a wide spectrum of pharmaceutical and biological products aimed at managing infections such as HIV, syphilis, gonorrhea, chlamydia, herpes, and HPV. Treatments include antibiotics, antivirals, vaccines, and topical formulations. The product landscape is evolving with rising antimicrobial resistance, prompting the development of novel antibiotics, long-acting injectables, and immunotherapies. Combination therapies and single-dose oral regimens are gaining popularity for improving adherence and reducing relapse.
Vaccines against HPV and herpes are key preventive components of the market. Diagnostic-linked therapies and digital adherence platforms are increasingly integrated into treatment ecosystems. With the WHO’s global initiatives toward STD elimination, pharmaceutical companies are investing heavily in targeted, broad-spectrum, and resistance-proof products to address unmet needs.
Key Takeaways
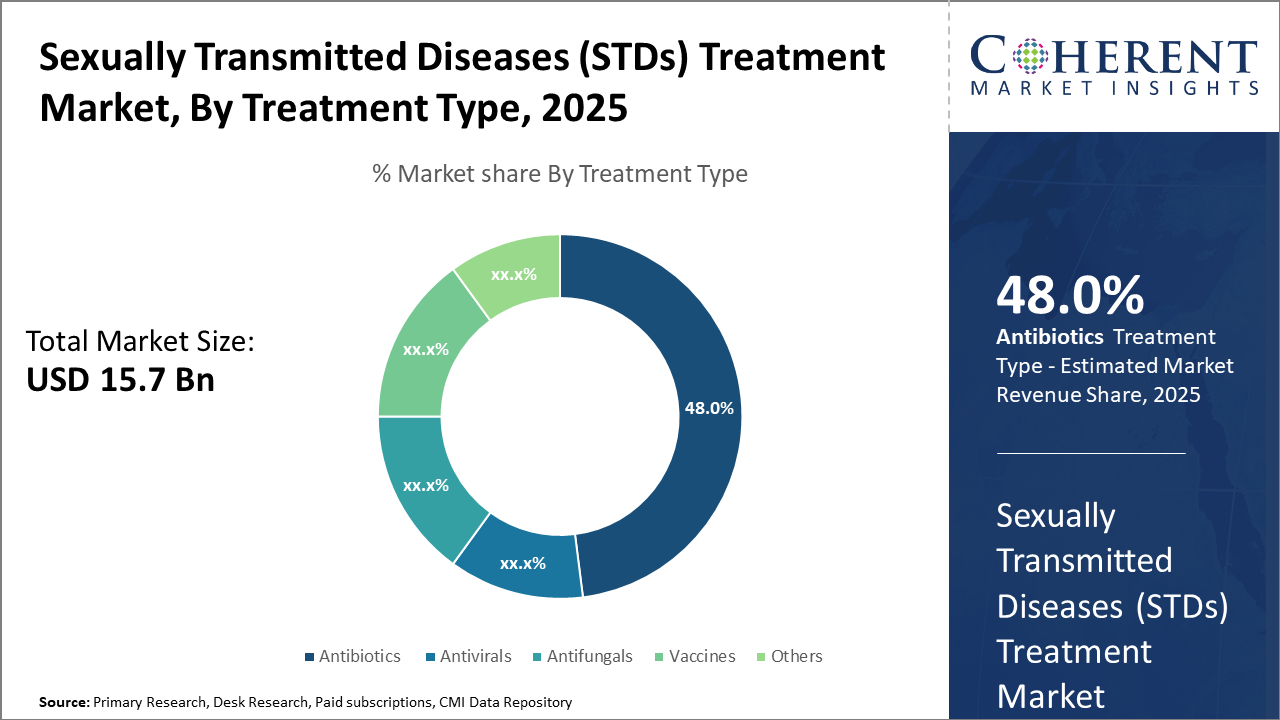
The antibiotics subsegment dominates the treatment type segment with a market share of 48%, driven by its efficacy in addressing bacterial STDs like chlamydia and syphilis.
Hospitals remain the principal end-user segment, accounting for 42% market share, supported by advanced diagnostic infrastructure and better treatment protocols.
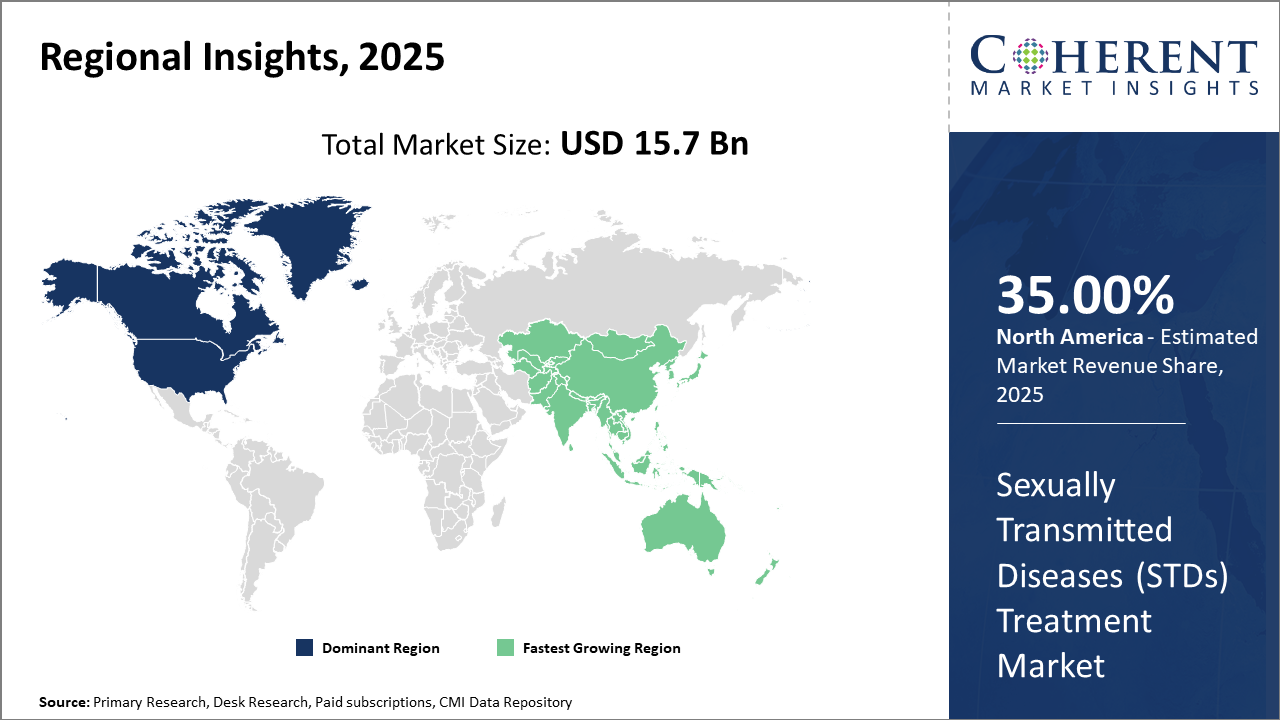
Among regions, North America leads with a robust industry share attributed to large-scale government programs and investments, while Asia Pacific exhibits the fastest CAGR driven by expanding healthcare access and rising STD cases.
European markets benefit from stringent regulatory frameworks that facilitate innovative drug approvals, creating further business growth opportunities.
Sexually Transmitted Diseases (STDs) Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
Sexually Transmitted Diseases (STDs) Treatment Market Insights, By Treatment Type
Antibiotics dominate the market share. Antibiotics hold a commanding lead due to their proven efficacy against common bacterial STDs such as chlamydia and syphilis, with advancements improving dosage regimens and limiting resistance. The Antibiotics subsegment benefits from continuous innovation targeting multidrug-resistant organisms, which is a principal growth factor. Antivirals are the fastest-growing subsegment, catalyzed by the increasing prevalence of viral STDs like HPV and herpes, and breakthroughs in long-acting formulations enhancing compliance. Antifungals maintain moderate growth driven by niche fungal STD infections treated primarily in specialized centers.
Sexually Transmitted Diseases (STDs) Treatment Market Insights, By Disease Type
Bacterial Infections dominate the market share. This dominance stems from higher case volumes and extensive treatment protocols requiring antibiotics. Recent surges in gonorrhea cases exhibiting antimicrobial resistance have prompted demand for novel therapeutics, intensifying market revenue from this subsegment. Viral Infections represent the fastest-growing subsegment, propelled by the rising burden of HSV and HIV/AIDS globally, coupled with innovative antiviral drugs and long-acting injectables entering clinical use. Parasitic Infections remain a smaller yet consistent segment, with targeted antiparasitic treatments catering to specific geographies.
Sexually Transmitted Diseases (STDs) Treatment Market Insights, By End-User
Hospitals dominate the market share. Hospitals possess advanced diagnostic capabilities and offer comprehensive treatment regimens, accounting for the largest revenue segment. The growing number of hospital-based STD clinics enhances market penetration, underpinned by government funding and insurance coverage. Clinics represent the fastest-growing subsegment, driven by expanding outpatient services and integrated community health programs, improving access. Diagnostic Centers witness steady growth due to increased screening demands and point-of-care testing innovations.
Sexually Transmitted Diseases (STDs) Treatment Market Trends
Market trend analysis reveals that personalized medicine and digital health platforms have rapidly transformed the Sexually Transmitted Diseases (STDs) Treatment market.
The rise in multidrug-resistant STD strains has spurred accelerated approvals for novel combination therapies in 2024, exemplified by the U.S. FDA’s fast-track clearance of new gonorrhea treatments.
Additionally, telemedicine’s incorporation in patient management resulted in over 30% of STD-related consultations being virtual in North America during the last year, improving treatment adherence.
Sexually Transmitted Diseases (STDs) Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Sexually Transmitted Diseases (STDs) Treatment Market Analysis and Trends
North America is the dominating region, holding over 35% of the market share, supported by comprehensive healthcare policies, high R&D investment, and expanded screening programs.
Asia Pacific Sexually Transmitted Diseases (STDs) Treatment Market Analysis and Trends
The Asia Pacific region is the fastest-growing market with a CAGR exceeding 9%. Government initiatives in India and China, expanding healthcare infrastructure, coupled with rising STD prevalence, facilitate this rapid growth. Key players such as Pfizer and Roche have intensified operations in these regions, focusing on combinations of product launches and digital interventions, enhancing market ecosystems, and fostering robust business growth.
Sexually Transmitted Diseases (STDs) Treatment Market Outlook for Key Countries
United States Sexually Transmitted Diseases (STDs) Treatment Market Analysis and Trends
USA Sexually Transmitted Diseases (STDs) Treatment Market Analysis and Trends
The USA remains the largest national market globally, driven by advanced healthcare infrastructure and high public expenditure on STD management. In 2024, approximately 2.5 million new STD cases were reported, fueling demand for effective treatments. Leading companies like Johnson & Johnson and Merck have launched innovative antiviral and antibiotic therapies targeted at resistant infections, acquiring significant market share. Further, reconstruction of reimbursement models favoring preventive treatment has propelled industry revenues.
India Sexually Transmitted Diseases (STDs) Treatment Market Analysis and Trends
India’s sexually transmitted diseases treatment market is rapidly expanding, typified by increasing government healthcare investments and rising STD incidence rates. Public health programs increased funding by 14% in 2024, focusing on rural outreach and awareness. Dominated by generic drug manufacturers like Cipla and Sun Pharmaceutical, India supplies low-cost antibiotics and antivirals regionally and globally. Urbanization and improved diagnostic availability have accelerated treatment uptake. Strategic collaborations between multinational companies and domestic manufacturers have intensified, enabling broader product availability and fostering sustained market revenue growth.
Analyst Opinion
A pivotal driver shaping market size is the elevated investment in novel antibiotic and antiviral therapies. Global pharmaceutical firms enhanced R&D spending by nearly 12% in 2024 compared to 2023, with new drug candidates for chlamydia and gonorrhea entering late-stage clinical trials. This trend supports increased production capacity projections for effective agents targeting drug-resistant strains.
From a demand perspective, expanding preventive screening programs across North America and Europe bolsters market revenue significantly. In 2024, public health campaigns led to a 15% rise in routine STD screenings, directly correlating with increased treatment uptake rates—especially for syphilis and herpes simplex virus infections.
On the micro-level, pricing dynamics show a shift towards value-based reimbursement models. Reimbursement changes implemented in the U.S. Medicare program in early 2025 prioritized outcomes-driven payments for chronic STD management, encouraging more sustainable market growth pathways.
Exports of generic antibiotics from major producers in India and China surged by over 18% YoY in 2024, reflecting both cost advantages and rising demand in emerging markets, which serves as a dynamic market driver boosting international reach and competitiveness.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: |
USD 15.7 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.3% | 2032 Value Projection: | USD 25.4 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Pfizer Inc., GlaxoSmithKline plc, Johnson & Johnson, Abbott Laboratories, Merck & Co., Inc., Teva Pharmaceuticals, Roche Holding AG, Novartis AG, Bayer AG, Hologic, Inc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Sexually Transmitted Diseases (STDs) Treatment Market Growth Factors
Increasing incidence and diagnosis of STDs worldwide due to improved surveillance and awareness programs, as evidenced by a 10% annual rise in reported cases in North America in 2024.Rising adoption of advanced treatment regimens and combination treatments, supported by breakthrough approvals such as novel antiviral drugs entering the market in 2025. Expansion of healthcare infrastructure, particularly in the Asia Pacific and the Middle East & Africa, enables wider patient access to treatments. Public health investment in India surged over 14% in 2024, enhancing STD management services. Growing emphasis on personalized medicine and digital health platforms facilitates patient adherence and continuous monitoring, improving clinical outcomes dramatically.
Sexually Transmitted Diseases (STDs) Treatment Market Development
In October 2025, Sunshine Biopharma launched a therapy using doxycycline targeting STDs, acne, and Lyme disease, introducing a 100 mg tablet formulation distributed across Canada through pharmacies and hospitals. The product is manufactured under Health Canada regulatory standards and positioned to broaden Sunshine’s presence in anti-infective therapies.
In March 2024, the American Medical Association (AMA) launched an online toolkit aimed at increasing routine screenings for HIV, STIs, viral hepatitis, and latent tuberculosis (LTBI). The toolkit is designed to help physicians and health care professionals implement screening workflows in clinics, emergency departments, and community health centers, with best practices for patient intake, linkage to care, and protocol standardization.
Key Players
Leading Companies of the Market
Pfizer Inc.
GlaxoSmithKline plc
Abbott Laboratories
Merck & Co., Inc.
Teva Pharmaceuticals
Roche Holding AG
Novartis AG
Bayer AG
Hologic, Inc.
Several market players have adopted vigorous growth strategies focused on portfolio diversification and innovative drug development. For instance, Pfizer’s collaboration with biotechnology firms to develop long-acting injectable treatments for herpes has enhanced its pipeline robustness, leading to an 8% revenue upscale in 2024. Similarly, Roche’s strategic acquisitions in molecular diagnostics have expanded its footprint in predictive STD diagnostics, facilitating improved treatment outcomes and stronger competitive positioning.
Sexually Transmitted Diseases (STDs) Treatment Market Future Outlook
The future STD treatment market will focus on innovation in long-acting injectables, resistance-proof antibiotics, and immune-based therapies. Advances in mRNA vaccine technology are expected to yield new preventive options beyond HPV and hepatitis. The integration of AI-driven diagnostics and telemedicine will streamline early detection and remote prescription, improving global disease management. Pharmaceutical pipelines are expanding with broad-spectrum antivirals and gene-based therapies that target latent infections. Growing governmental funding for sexual health programs, coupled with rising awareness in emerging regions, will drive market penetration. As digital health ecosystems mature, STD treatment delivery will increasingly merge with virtual care, enabling real-time monitoring and adherence optimization.
Sexually Transmitted Diseases (STDs) Treatment Market Historical Analysis
The STD treatment market has undergone a major transformation over the past decades, driven by medical advancements and public health initiatives. Initially reliant on broad-spectrum antibiotics, the market evolved as diagnostic precision improved and specific pathogens were better characterized. The HIV epidemic in the 1980s reshaped global attention toward sexually transmitted infections, spurring the development of antiretroviral therapies and awareness campaigns. The early 2000s witnessed the introduction of single-dose antibiotic regimens for chlamydia and gonorrhea, alongside increased vaccine development for HPV. However, the emergence of antibiotic resistance in gonococcal strains and viral persistence in herpes and HIV infections presented ongoing challenges. By the late 2010s, combination therapies, pre-exposure prophylaxis (PrEP), and digital health interventions improved accessibility and adherence rates.
Sources
Primary Research Interviews:
Infectious Disease Specialists
Public Health Officials
Pharmacologists
Epidemiologists
Databases:
WHO Global HIV & STI Surveillance
CDC STD Data
ClinicalTrials.gov
Magazines:
Medical News Today
The Lancet Infectious Diseases
Pharma Times
Healthline Medical
Journals:
Sexually Transmitted Diseases
Journal of Infectious Diseases
Clinical Infectious Diseases
PLOS Pathogens
Associations:
World Health Organization (WHO)
Centers for Disease Control and Prevention (CDC)
International Union against Sexually Transmitted Infections (IUSTI)
Infectious Diseases Society of America (IDSA)
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients