Sarcoma Treatment Market Size and Forecast – 2025 – 2032
The Global Sarcoma Treatment Market size is estimated to be valued at USD 4.7 billion in 2025 and is expected to reach USD 7.9 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 7.6% from 2025 to 2032.
Global Sarcoma Treatment Market Overview
Sarcoma treatment products include pharmaceutical and therapeutic interventions used to treat soft tissue and bone sarcomas. These products comprise chemotherapy drugs, targeted therapies, immunotherapies, radiation therapy systems, and surgical tools used in tumor resection. Drug products vary by mechanism of action, including cytotoxic agents, kinase inhibitors, and monoclonal antibodies. Treatment products are often used in combination regimens and tailored based on sarcoma subtype, tumor location, and disease stage.
Key Takeaways
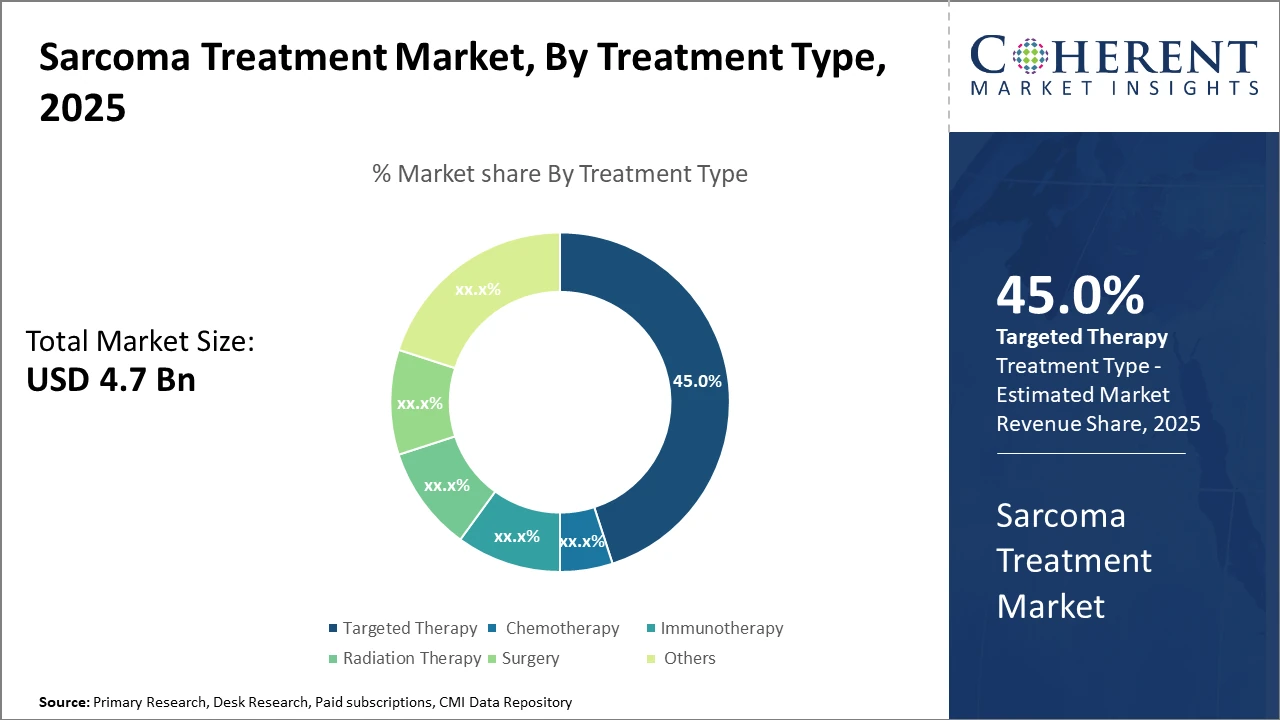
The Targeted Therapy segment dominates the Sarcoma Treatment Market with 45% market share, primarily driven by new molecular agents and kinase inhibitors improving treatment specificity.
Soft Tissue Sarcoma remains the largest sarcoma type segment, attributed to its higher prevalence and more diverse treatment pipeline.
Hospitals continue to be the primary end-user segment due to the need for multidisciplinary care and complex therapeutic procedures.
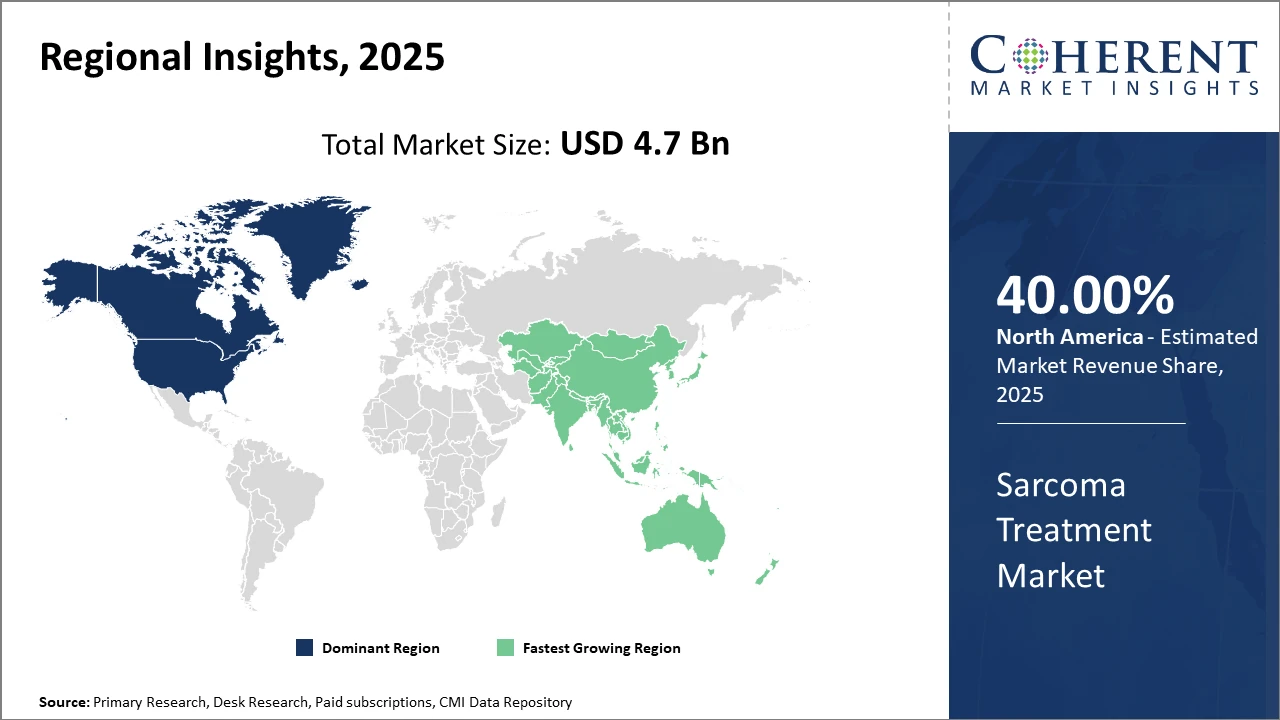
Regionally, North America holds a commanding market share, benefiting from advanced healthcare infrastructure and early adoption of innovative therapies.
Meanwhile, Asia Pacific emerges as the fastest-growing region, propelled by increased healthcare spending, favorable government policies supporting oncology research, and expanding access to novel drugs.
Sarcoma Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
Sarcoma Treatment Market Insights, By Treatment Type
Targeted Therapy leads the market due to its highly specific mode of action on molecular pathways, offering improved efficacy and reduced toxicity compared to traditional therapies. The growing pipeline of tyrosine kinase inhibitors and small-molecule agents in 2024 has significantly contributed to this dominance. Immunotherapy is the fastest-growing segment, driven by successful checkpoint inhibitors and adoptive cell therapies emerging from recent clinical successes, particularly in refractory sarcoma cases. Chemotherapy remains an essential option for certain sarcoma types despite challenges with side effects and resistance. Radiation Therapy and Surgery continue to hold steady shares owing to their established role in localized disease management. The Others segment includes emerging modalities such as photodynamic therapy or gene therapy in early experimental stages.
Sarcoma Treatment Market Insights, By Sarcoma Type
Soft Tissue Sarcoma maintains leadership due to its higher incidence and heterogeneous subtypes requiring diverse treatment regimens, supported by targeted and immunotherapeutic innovation. Bone Sarcoma, specifically osteosarcoma, is the fastest-growing subsegment, fueled by new treatment approvals and improved diagnostic tools facilitating earlier intervention. GIST also sees steady growth because of effective molecularly targeted drugs, while Kaposi’s Sarcoma holds a smaller but significant share associated with HIV-related cases. The Others segment includes rare sarcoma subtypes that remain a research focus for future therapeutic development.
Sarcoma Treatment Market Insights, By End-User
Hospitals are favored for sarcoma treatment due to their multidisciplinary capabilities, advanced surgical facilities, and access to comprehensive oncology services. Specialty Clinics provide focused outpatient care and are expanding in niche therapeutic administration, though at a smaller scale. Research Institutes play a critical role in clinical trials and pipeline development, but contribute to a limited direct market share. Ambulatory Surgical Centers offer convenience and cost benefits for select procedures but remain a niche segment. The Others category entails home care and telehealth services supporting follow-up treatment, reflecting emerging patient management trends.
Sarcoma Treatment Market Trends
Recent market trends emphasize the pivotal role of targeted therapies, supported by an increasing number of FDA approvals in 2024, such as kinase inhibitors for soft tissue sarcoma.
Immunotherapy agents, including checkpoint inhibitors, are gaining traction with early phase trial successes showing improved survival rates in select sarcoma populations.
Additionally, the integration of digital health tools aids in real-time treatment response monitoring, enhancing personalized care frameworks.
For example, remote patient management platforms launched in North America in 2023 have demonstrated a 20% improvement in treatment adherence.
Sarcoma Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Sarcoma Treatment Market Analysis and Trends
In North America, the dominance in the market arises from substantial healthcare expenditure, advanced research capabilities, and early adoption of innovative therapies. The U.S. accounts for nearly 40% of the total market share, with significant contributions from drug approvals and comprehensive treatment centers. Regulatory incentives and reimbursement reforms further strengthen market dynamics.
Asia Pacific Sarcoma Treatment Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR of approximately 9.3%, driven by growing healthcare infrastructure, increased sarcoma awareness, and government initiatives to promote oncology research. China and India stand out given their rising incidence rates and expanding clinical trial activities. Market players' increasing investments in these regions highlight the strategic significance.
Sarcoma Treatment Market Outlook for Key Countries
USA Sarcoma Treatment Market Analysis and Trends
The USA’s market benefits from robust clinical trial ecosystems with over 50 ongoing phase II and III trials in 2024 targeting rare sarcoma subtypes. Industry leaders have strategically partnered with research institutes to accelerate novel drug development. Biopharma giants like Pfizer and Novartis have contributed substantially to new molecular entities approved recently, solidifying the U.S. as a global innovation hub. Enhanced insurance coverage for novel therapies and precision diagnostics continues to propel market growth forward.
Japan Sarcoma Treatment Market Analysis and Trends
Japan’s market is characterized by supportive regulatory policies that facilitate rapid drug approval and reimbursement for orphan diseases, including sarcoma. Leading domestic players have intensified research on immunotherapies specifically tailored to Asian genetic profiles, bolstering treatment efficacy. Government-led cancer control programs and collaborations with multinational companies have also expanded access to novel therapeutics, enhancing patient outcomes.
Analyst Opinion
The surge in innovative targeted therapies remains a pivotal market growth driver. Recently, the approval of several kinase inhibitors and immunotherapies in 2024 alone has expanded patient options, leading to a notable increase in market revenue. For instance, advancements in precision oncology indicated a 15% year-over-year increase in patient enrollment for clinical trials exploring novel sarcoma treatments.
The increasing prevalence of sarcoma cases worldwide, particularly soft tissue and bone sarcomas, is creating substantial demand. Data from 2024 estimates approximately 13,000 new sarcoma cases in the U.S., with a proportionate rise across Europe and the Asia Pacific, resulting in expanded treatment scope and boosting market share for new entrants.
Growing adoption of multimodal treatment strategies, including surgery, radiation, and systemic therapies, is reshaping treatment protocols. Real-world evidence from oncology centers in Europe reveals that combination regimens have improved progression-free survival by approximately 18%, positively impacting overall market growth.
Supply-side dynamics, such as expanded manufacturing capacities for biologics and small molecules catering to sarcoma treatments, are improving drug availability and pricing stability. Recent capacity expansions in pharmaceutical hubs across North America in 2024 have reduced lead times for new drug launches, corroborating increased market revenue potential.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 4.7 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.6% | 2032 Value Projection: | USD 7.9 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Bristol-Myers Squibb, Roche Holding AG, Daiichi Sankyo Company, Limited, Astellas Pharma Inc., Epizyme, Inc., Blueprint Medicines Corporation, PharmaMar S.A., Incyte Corporation, Amgen Inc., Takeda Pharmaceutical Company Limited | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Sarcoma Treatment Market Growth Factors
Multiple catalysts are fuelling robust growth across the sarcoma treatment market. Firstly, heightened R&D investments in novel targeted therapies have unlocked potent treatment modalities addressing previously refractory subtypes. The growing incidence of sarcoma, particularly in aging populations and urbanized regions, drives increasing demand for effective therapies. Additionally, technological advancements in diagnostic imaging and molecular profiling improve early detection rates, directly impacting treatment efficacy and patient outcomes. Apart from this, evolving reimbursement policies and expanded healthcare infrastructure in emerging economies have enhanced market accessibility, contributing to sustained market growth.
Sarcoma Treatment Market Development
In August 2024, the U.S. FDA approved Tecelra (afami-cel), the first engineered cell therapy for adults with synovial sarcoma, marking a major breakthrough in solid tumor treatment. The approval represented a significant advancement for adoptive cell therapies beyond hematologic cancers, offering a targeted option for patients with advanced, unresectable disease.
In January 2024, the U.S. FDA approved Merck KGaA’s Carcinostat for the treatment of Ewing sarcoma, expanding therapeutic options for this rare and aggressive bone and soft-tissue cancer. The approval strengthened Merck’s oncology portfolio and addressed unmet needs in pediatric and young adult sarcoma care.
Key Players
Leading Companies of the Market
Bristol-Myers Squibb
Roche Holding AG
Daiichi Sankyo Company, Limited
Epizyme, Inc.
Blueprint Medicines Corporation
PharmaMar S.A.
Incyte Corporation
Amgen Inc.
Takeda Pharmaceutical Company Limited
Leading companies have adopted aggressive strategies, including strategic acquisitions, collaborative R&D partnerships, and extensive clinical trial investments. For example, Pfizer’s acquisition of a key immunotherapy asset in 2023 expanded its pipeline significantly, resulting in a 12% surge in sarcoma treatment-related revenue. Similarly, Epizyme's co-development agreements have accelerated its epigenetic inhibitor trials, with faster market access expected from 2025 onward.
Sarcoma Treatment Market Future Outlook
The future outlook for the sarcoma treatment market is shaped by advances in precision medicine and immunotherapy. Increasing investment in rare cancer research, expanded clinical trials, and regulatory incentives are expected to accelerate innovation. Combination therapies and personalized treatment regimens will improve patient outcomes and drive market expansion. Global collaboration in oncology research will further enhance therapeutic development.
Sarcoma Treatment Market Historical Analysis
The sarcoma treatment market historically faced challenges due to the rarity and diversity of sarcoma subtypes. Treatment options were limited to surgery, chemotherapy, and radiation, with variable outcomes. Over time, improved diagnostic techniques and greater clinical research focus helped refine treatment strategies. The introduction of targeted therapies marked a significant advancement, improving outcomes for specific sarcoma types and strengthening market growth
Sources
Primary Research Interviews:
Oncologists
Clinical Trial Investigators
Hospital Pharmacists
Cancer Researchers
Biotech Executives
Databases:
NIH Cancer Data
WHO Oncology Statistics
ClinicalTrials.gov
Magazines:
Oncology Times
Cancer Today
Pharma Intelligence
BioCentury
Medical News Today
Journals:
The Lancet Oncology
Journal of Clinical Oncology
Cancer Research
Sarcoma Journal
Nature Reviews Cancer
Associations:
American Cancer Society
European Society for Medical Oncology
National Cancer Institute
WHO
Sarcoma Foundation
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients