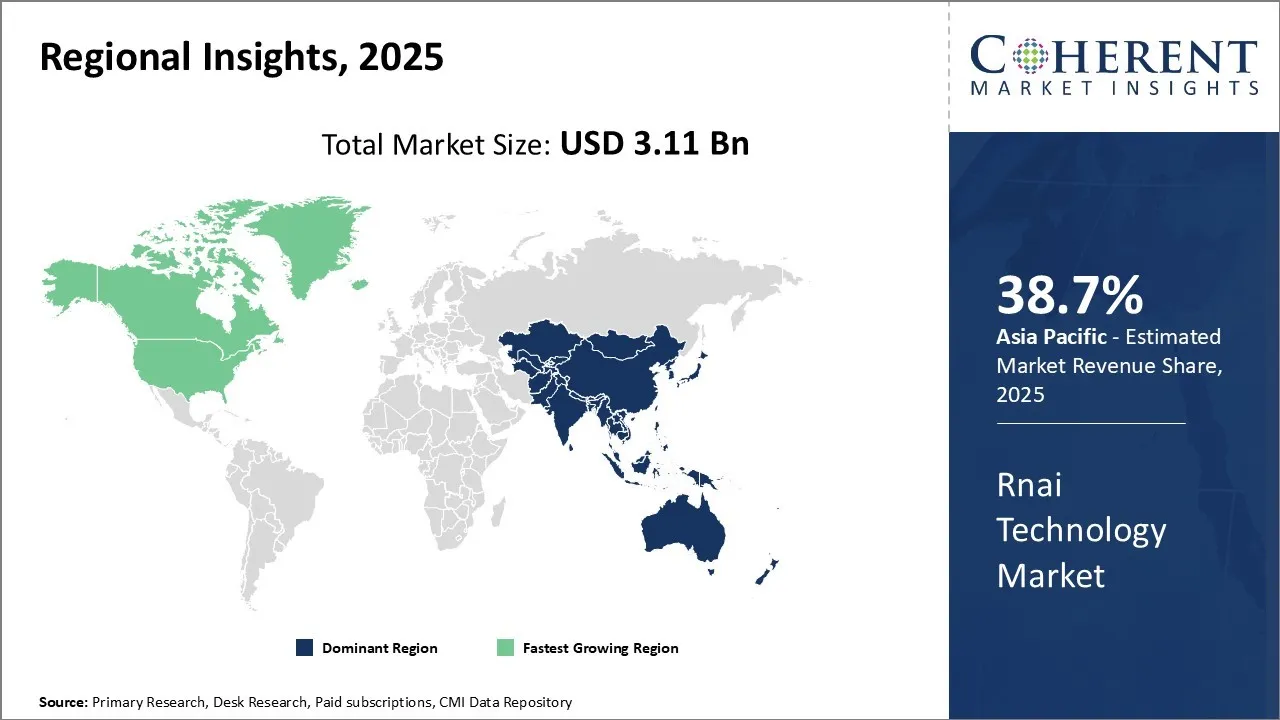
Global RNAi technology market report is estimated to be valued at USD 3.11 Bn in 2025 and is expected to reach USD 8.27 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 15% from 2025 to 2032.
To learn more about this report, Download Free Sample
The development of improved RNAi delivery mechanisms and next generation targeting capabilities can boost demand. More pharmaceutical companies are leveraging RNAi for drug discovery and developing RNAi therapeutics for genetic disorders, oncology, and infectious diseases. Increased funding for R&D and growing clinical trial approvals are can provide opportunities for the market growth. Continued expansion of CRISPR and other gene editing technologies hamper RNAi technology adoption in the future.
|
Current Events |
Description and its Impact |
|
Advancements in RNAi delivery systems |
|
|
Collaborations and partnerships |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
The integration of artificial intelligence is gradually transforming the RNA interference technology demand. However, the primary and, predominant change that is observed recently is the capability of AI to provide with high-quality, single-cell datasets which helps in developing advanced pipelines for data harmonization and ensuring human-in-the-loop validation. This modern integration also empowers researchers to accelerate RNAi therapeutics development. This has resolved the challenges faced by researchers including low-quality public datasets, inconsistent annotations, and lack of structured storage systems form gene-silencing condition, all these issues are creating a time-consuming and resource intensive hustle. For instance, in January 2025, Elucidata, the most recognized biotech company is leveraging their expertise in data harmonization and annotation by collaborating with leading biopharma organizations to accelerate discovery process, reduce validation times and to enhance precision of gene identification for RNAi therapeutics.
By application, drug discovery and development segment are estimated to contribute the highest market share of 40.7% in 2025 due to the versatility of RNAi technologies. RNA interference allows researchers to efficiently study gene function and validate drug targets. This process of discovering the roles of specific genes in health and disease is profoundly accelerated using RNAi techniques as compared to other molecular biology methods. RNAi technologies have become a mainstream approach for high-throughput screening of potential drug targets. By knocking down gene expression, scientists can evaluate a gene's involvement in various disease mechanisms. This enables the selection and prioritization of promising drug targets early in the drug discovery pipeline. RNAi technologies are also being utilized to identify new biomarkers and develop personalized medicines. The ability to control gene expression with RNAi can offer new therapeutic strategies for previously undruggable targets. As biopharmaceutical companies work to overcome challenges in drug discovery like high attrition rates, the flexibility and throughput provided by RNAi technologies makes them an indispensable asset. RNAi allows the high-volume evaluation of novel targets and rapid validation of therapeutic hypotheses. Customized siRNA and shRNA libraries created for specific disease pathways further enhance the drug discovery potential.
In February 2025, Alnylam Pharmaceutical Inc. the leading RNAi therapeutics company hosted an R&D Day in New York. The company showcased it R&D progress and platform innovations, including updates in multiple near and mid-stage potentially transformative therapies the represent blockbuster opportunities as its pipeline rapidly expands across various therapeutic areas.
By end user, pharmaceutical and biotechnology companies’ segment is estimated to contribute the highest market share of 50.6% in 2025 due to the expanding therapeutic applications of RNAi. RNAi-based drugs offer promising new treatments for various inherited disorders and cancers by selectively silencing disease-causing genes. A major driver is the approval and commercialization of the first-ever RNAi therapeutic, Patisiran, to treat hereditary transthyretin-mediated amyloidosis. More RNAi drugs are progressing through clinical trials for conditions such as alpha-1 antitrypsin deficiency, transthyretin amyloidosis, primary hyperoxaluria type 1, and others. The arrival of new RNAi therapeutics are expected to strengthen pharmaceutical leadership as these innovative medicines reach the market. Growing interest in RNAi as a platform technology paves the way for various new modalities such as nucleotide prodrugs, GalNAc-siRNA conjugates, lipid nanoparticles, and more. These advancements enhance delivery capabilities while maintaining potency and safety.
In February 2025, OliX Pharmaceutical, Inc., South Korea based leading developer of RNAi therapeutics announced a global licensing agreement with Eli Lilly and Company. This collaboration focuses on the development and commercialization of OliX’s OL75016, a phase 1 candidate primarily targeting metabolic-associated steatohepatitis (MASH) and other cardiometabolic indications.
To learn more about this report, Download Free Sample
The Asia Pacific region is increasingly becoming a dominant force in the global RNAi technology market, with high share of 38.7% in 2025, primarily driven by soaring investments in research and development related to RNAi therapeutics and a growing focus on personalized medicine. Robust government initiatives and funding, strategic collaborations among biotech and pharmaceutical companies, and the rising prevalence of chronic diseases like cancer and hepatitis B, which require novel therapeutic approaches, further bolster this growth. For instance, in March 2024, UNSW Sydney Received an investment for USD 40 million from the Federal Government to grow Australia’s global presence in science with RNA vaccines and therapies. Countries such as Japan, China, and South Korea are making significant advancements in RNAi research, harnessing the potential of these technologies for developing innovative treatments.
For instance, in April 2025, Ono Pharmaceuticals Japan-based company, collaborated with US-based startup Jorna Therapeutics. This partnership is expected to accelerate efforts to bring RNA editing-based therapies to market by leveraging Ono's global resources and expertise, Additionally, the Asia Pacific region benefits from cost-effective labor and manufacturing processes, increasing international partnerships, and a thriving academic and clinical research environment, which are pivotal to the growth of the RNAi technology market.
North America exhibits the fastest growth rate in the RNAi technology market. This trend can be attributed to several factors, including the presence of a sophisticated healthcare infrastructure, substantial biotechnology and pharmaceutical industries, and a strong focus on research and innovation. The region's regulatory landscape is conducive to the development and approval of advanced therapeutics, and with substantial investments in genomics and proteomics, there is an accelerated uptake of RNAi technologies in drug development processes. North America’s advanced clinical trial ecosystem. For instance, in May 2025, researchers at the University of Minnesota completed a first-in-human clinical trial testing a CRISPR/Cas9 gene-editing technique to help the immune system fight advanced gastrointestinal (GI) cancers. Another driver is its significant intellectual property rights provisions, and presence of key industry players contribute to its rapid progress in the RNAi technology sector. Injecting substantial resources into tackling genetic disorders and life-threatening diseases, North America continues to pave the way for advancements in RNAi therapeutics and diagnostics, setting a precedent for RNAi technology market growth globally.
China is the leading country in the Asia Pacific region in RNAi technology market. The expanding pipeline for China is observed to be due to the highest rate of drug candidates in development has quintupled, Chinese companies are focusing on innovative drugs leading to the approval of 30 medicines discover in China 2023, accounting for 37% of new drug approved in China. Along with that China also has a strong emphasis on biologics. China is investing heavily in next-generation technologies such as bispecific antibodies, cell therapies, and antibody-drug conjugates. China has become the powerhouse of biologics with over 100 biosimilars in clinical development and more than 40 projects coming up in the market. This further fuels the RNAi technology market revenue.
The United States is the leading country in the North American region regarding RNAi technology market. The United States is primarily known for its strong research and development infrastructure, robust biotechnology sector in the U.S. The biotechnology industry is a major economy driver in the United States, generating approximately USD 183 billion in revenue in 2024. At present, the U.S. biotechnology companies employ over 431,600 people but there is a rise in demand for skilled professionals. This is further propelling the RNAi technology market demand.
RNAi, a gene silencing technique based on dsRNA, provided a platform for genetic alternations aimed for improving crops by changing them for essential agronomical trait. RNAi is a highly conserved gene regulatory system which regulates gene expression at the posttranscriptional stage. It also has the ability to target particular gene in pests and pathogens making it a valuable tool for developing a sustainable and environmentally friendly agricultural practices.
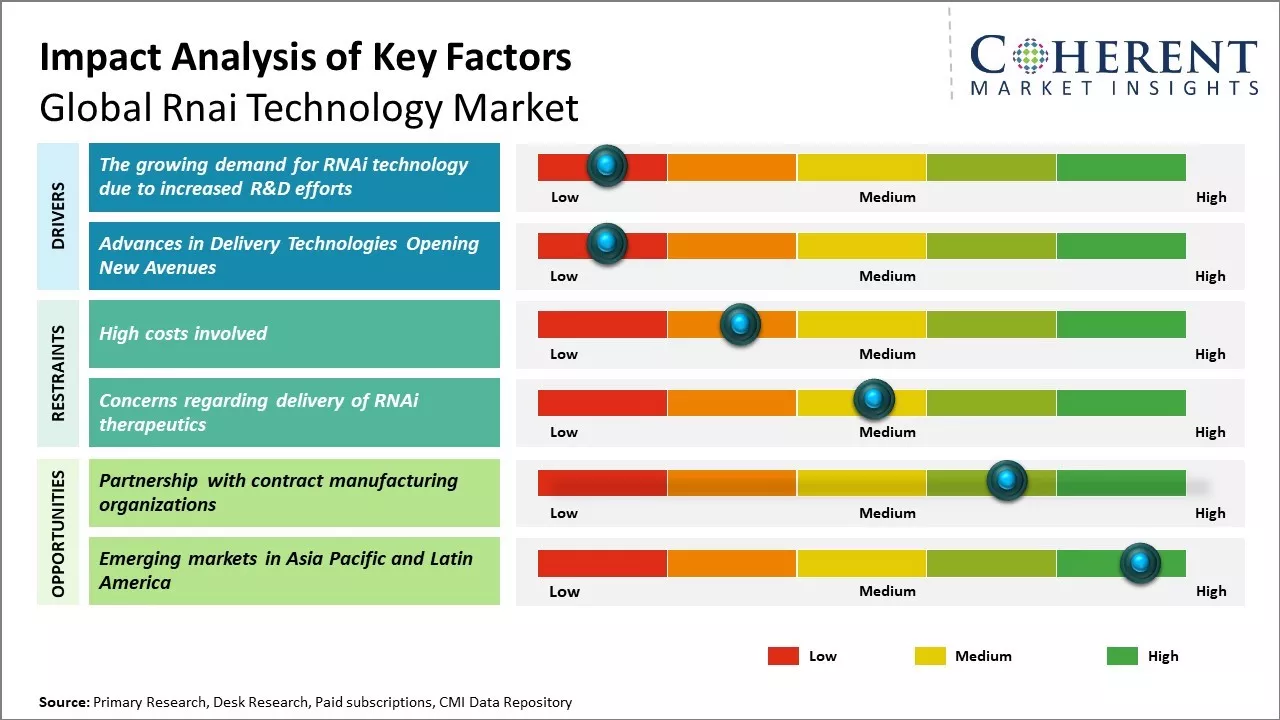
The rising demand for RNAi technology due to increased research and development efforts by pharmaceutical companies and research institutions can drive the market growth. RNAi refers to the process of gene silencing through the introduction of double-stranded RNA molecules into the cell, which helps in selectively turning off specific genes. This mechanism is being actively explored by drug developers for its potential to develop powerful new therapies for various diseases. Many biotech companies and academic research labs across the globe are demonstrating the broad potential of RNAi technology through various preclinical and clinical research studies. In November 2020, Alnylam Pharmaceuticals, Inc. introduced new Value-based Agreements (VBAs) aimed at enhancing patient access to givosiran, thus, strengthening the company's position in RNAi therapeutics and bolstering its business prospects. In December 2021, Novartis AG obtained approval from the U.S. Food and Drug Administration for Leqvio, the first and only small interfering RNA (siRNA) therapy that is designed to reduce low-density lipoprotein levels with just two doses per year: an initial dose followed by another at three months.
One of the major bottlenecks limiting the clinical potential of RNAi therapeutics has been the lack of efficient and safe delivery systems. The highly labile nature of nucleic acid drugs and challenges associated with their in vivo delivery to target sites requires innovative formulation and design strategies. Novel delivery technologies such as ligand-conjugated nanoparticles, exosome-mimetic vesicles and cell-penetrating peptides can enhance the cellular uptake and protection of RNAi payloads. Advancements are also being made in modulating biodistribution through tissue-targeting ligands and optimizing pharmacokinetic profiles. Recent delivery platforms enable targeting of RNAi molecules to specific organs, cell types and subcellular locations based on pathological needs. This high precision delivery promises to augment therapeutic indices while reducing adverse effects. As more biocompatible carriers successfully navigate clinical translation, these will facilitate the development of RNAi drugs for diverse pathological conditions including those traditionally considered difficult to drug.
Partnering with contract manufacturing organizations could provide companies in the global RNAi technology market forecast with significant opportunities for growth. Contract manufacturers allow firms to focus on their core competencies of research and development while reducing capital expenditure requirements for manufacturing infrastructure and equipment. This allows smaller biotech companies working with limited capital to harness the scale and expertise of specialized CMOs. CMOs offer flexible, high-quality manufacturing capabilities tailored to the complex needs of RNAi production. RNAi therapeutics often utilize multi-step synthesis and purification methods that demand stringent quality control protocols to ensure product safety and efficacy. Established CMOs have deep experience adhering to current good manufacturing practices and can more easily meet regulatory expectations compared to in-house manufacturing facilities of smaller biotech companies. Partnering with CMOs experienced in mRNA, siRNA or other RNA production techniques allows firms to rely on optimized manufacturing know-how and capacity instead of building these capabilities from scratch internally.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3.11 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 15% | 2032 Value Projection: | USD 8.27 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Alnylam Pharmaceuticals, OriGene Technologies, Inc., Arrowhead Pharmaceuticals, Dicerna Pharmaceuticals, Silence Therapeutics, Ionis Pharmaceuticals, Merck & Co. , Qiagen N.V., Thermo Fisher Scientific, Synlogic, Benitec Biopharma, Gradalis, Sirnaomics, Inc., Marina Biotech, Quark Pharmaceuticals, Regulus Therapeutics, GeneCare Research Institute Co., Ltd., ASC Therapeutics |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: Global RNAi technology market involves the development and use of RNA interference techniques and platforms for various research and therapeutic applications. RNA interference utilizes small interfering RNA molecules to silence or alter targeted gene expression, showing promise for treating genetic disorders, infectious diseases, and various types of cancer. This market covers products and services focused on RNAi delivery mechanisms, libraries, design tools, and microarray systems for applications such as drug discovery, therapeutics development, agricultural research, and studying gene function. Key players are developing novel RNAi technologies to drive continued growth in this innovative space.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients