Risk-Based Monitoring Market Size and Forecast – 2025 – 2032
The Global Risk-Based Monitoring Market size is estimated to be valued at USD 1.8 billion in 2025 and is expected to reach USD 3.2 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 8.2% from 2025 to 2032.
Global Risk-Based Monitoring Market Overview
Risk-based monitoring products are software platforms and analytical tools used to oversee clinical trials, quality systems, and regulatory compliance activities. These solutions prioritize high-risk processes, sites, or data points using algorithms, real-time analytics, and centralized dashboards. Risk-based monitoring products help organizations optimize resource allocation, improve data integrity, and detect anomalies early. Features typically include automated alerts, statistical modeling, audit trails, and integration with clinical data management systems.
Key Takeaways
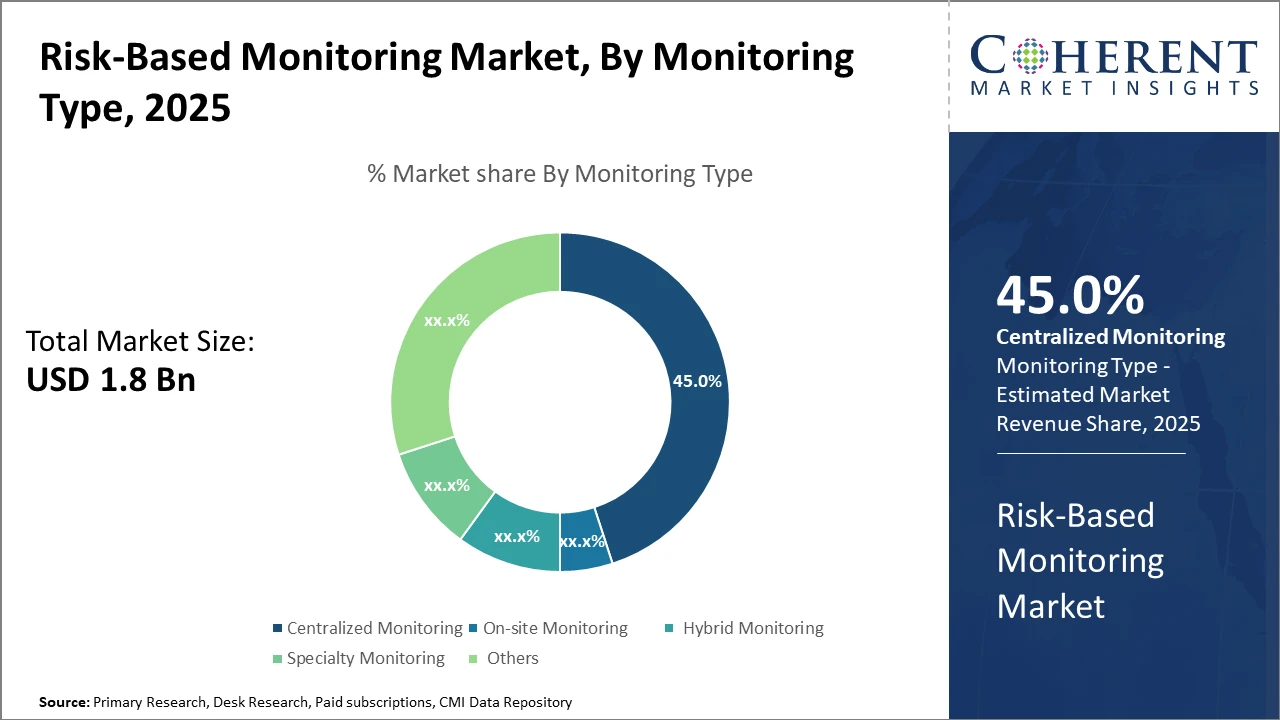
Centralized Monitoring leads the monitoring type segment with 45% market share due to its cost-efficiency and comprehensive oversight capabilities, while Hybrid Monitoring emerges as the fastest-growing approach driven by increasing demand for flexible monitoring solutions. Other forms, including Specialty Monitoring, continue to address niche clinical trial requirements.
Pharmaceutical companies dominate end-user adoption, reflecting their extensive clinical pipelines; academic and research institutes, however, are the fastest-growing end-user driven by expanding translational research efforts.
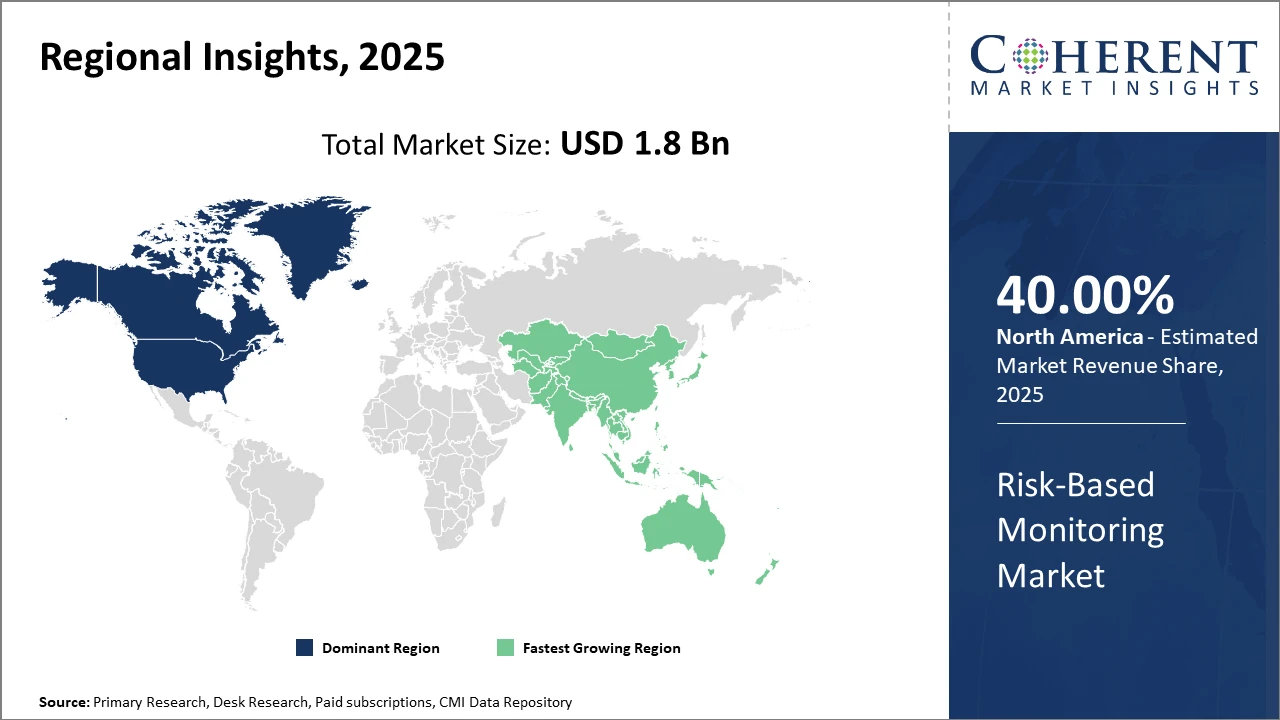
North America holds the largest market share thanks to regulatory frameworks and advanced healthcare infrastructure, while the Asia Pacific is the fastest-growing region, driven by rising clinical trial outsourcing and governmental initiatives.
Europe leverages stringent regulatory compliance, with notable collaborative efforts among market players to enhance RBM tools conforming to GDPR and other privacy laws.
Risk-Based Monitoring Market Segmentation Analysis
To learn more about this report, Download Free Sample
Risk-Based Monitoring Market Insights, By Monitoring Type
Centralized Monitoring dominates the market share with 45%, thanks to its ability to provide comprehensive remote oversight and cost reduction compared to traditional on-site approaches. The rise in complex multi-center studies has further consolidated demand for centralized solutions. Hybrid Monitoring is the fastest-growing subsegment, combining on-site visits with remote data analytics to ensure flexibility in risk mitigation while optimizing resource allocation. On-site Monitoring continues to address regulatory requirements and high-risk protocols, Specialty Monitoring targets specialized trials such as oncology, and Others cover emerging innovative monitoring methodologies.
Risk-Based Monitoring Market Insights, By End-User
Pharmaceutical companies command the largest market share due to their expansive global trial portfolios and significant R&D budgets. They deploy RBM to balance regulatory compliance with operational efficiency. Academic & Research Institutes are the fastest growing subsegment, driven by increasing translational research and federally-funded projects that demand risk-based compliance frameworks. CROs maintain steady growth by acting as intermediaries for multiple sponsors. Biotechnology firms show moderate uptake, focusing on early-phase clinical studies, while Others represent niche players adopting RBM for non-traditional clinical applications.
Risk-Based Monitoring Market Insights, By Deployment Models
Cloud-based deployment is rapidly gaining traction due to its scalability, lower upfront costs, and real-time collaborative capabilities, fostering increased adoption across diverse market players. This subsegment holds the position of fastest growth in deployment mode, propelled by enhanced data security protocols and ease of integration with other cloud-native eClinical systems. On-Premise solutions retain preference among organizations with stringent data governance policies or legacy system investments, while Web-Based platforms offer user-friendly interfaces suitable for smaller organizations and decentralized trials.
Risk-Based Monitoring Market Trends
The Risk-Based Monitoring landscape is increasingly driven by technological innovations such as machine learning algorithms and cloud computing, enabling dynamic risk identification and mitigation.
For instance, AI-enabled RBM platforms launched in 2024 offer predictive analytics that decreased monitoring visits by approximately 30%, enhancing efficiency.
Additionally, the rise in decentralized clinical trials facilitated by pandemic-induced digital healthcare acceleration further propels market evolution.
Pilots deploying AR for virtual site assessments have improved sponsor oversight without physical presence, exemplified by recent 2025 collaborations between technology providers and pharmaceutical sponsors.
These trends signify a market shift from traditional on-site monitoring paradigms toward integrated, technology-enabled frameworks.
Risk-Based Monitoring Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Risk-Based Monitoring Market Analysis and Trends
In North America, the dominance in the Risk-Based Monitoring market is attributed to vigorous clinical research activities, predominantly in the U.S., supported by a strong regulatory ecosystem including FDA guidelines promoting risk-based approaches. The presence of top-tier pharmaceutical companies and CRO headquarters further fuels demand. Approximately 40% of the market revenue is generated here as of 2025, driven by early technology adoption and extensive clinical trial volumes.
Asia Pacific Risk-Based Monitoring Market Analysis and Trends
Meanwhile, the Asia Pacific exhibits the fastest growth with a CAGR exceeding 10% from 2025 to 2032. Government-led initiatives to attract clinical trials, enhanced healthcare infrastructure, and expand CRO presence encourage RBM adoption. Countries like China and India are spearheading growth backed by increasing outsourcing and digital health integration, contributing to an evolving market ecosystem.
Risk-Based Monitoring Market Outlook for Key Countries
USA Risk-Based Monitoring Market Analysis and Trends
The USA's Risk-Based Monitoring market reflects significant expansion aided by early regulatory awareness and digital health investments. Leading industry participants have established advanced RBM centers equipped with AI-driven solutions, reducing trial cycle times by up to 25% in 2024. Collaborative initiatives between federal agencies and private entities have focused on standardizing RBM frameworks, propelling the country’s market share dominance. Additionally, extensive clinical trial volumes from biotech hubs in Massachusetts and California bolster demand.
China Risk-Based Monitoring Market Analysis and Trends
China's Risk-Based Monitoring market benefits from government incentives promoting innovation in clinical research oversight. The country has witnessed over 35% year-on-year growth in RBM deployments since 2023, driven by partnerships between local CROs and international pharmaceutical firms. Regulatory reforms aimed at harmonizing clinical trial standards with global guidelines have accelerated RBM adoption, supported by growing investments in telemedicine infrastructure and AI applications.
Analyst Opinion
Risk-Based Monitoring adoption is primarily driven by demand-side indicators such as increasing clinical trial participant numbers and escalating complexity of clinical protocols. In 2024, over 50% of large-scale Phase III trials incorporated RBM strategies, rising from 40% in 2022, according to clinical trial registries data, underscoring growing market share potential.
Supply-side dynamics highlight significant expansion of RBM tool production capacity, with global software deployments rising by 35% year-over-year in 2025. Pricing models have increasingly shifted toward subscription-based SaaS offerings, enhancing affordability and uptake among mid-sized clinical research organizations.
Diverse use case applications across the pharmaceutical, biotechnology, and medical devices sectors reveal growing RBM integration with artificial intelligence (AI) and remote monitoring technologies. For instance, in 2024, the use of AI-augmented RBM platforms increased trial site monitoring efficiency by over 25% as reported in industry conferences.
Micro-level indicators reflect regional variances, with North America and Europe leading in RBM market revenue due to stringent regulatory requirements. In contrast, Asia Pacific demonstrates nascent yet fastest-growing demand driven by government incentives and increasing clinical trial outsourcing activities.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.2 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.2% | 2032 Value Projection: | USD 3.8 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Syneos Health, PRA Health Sciences, Pharmaceutical Product Development (PPD), PharmaLex GmbH, eClinical Solutions, BenevolentAI, Capgemini, Indegene, Saama Technologies, Medable Inc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Risk-Based Monitoring Market Growth Factors
The growing complexity of clinical trials necessitates more sophisticated monitoring techniques, driving RBM market growth. In 2024, complex adaptive trial designs accounted for nearly 30% of newly initiated trials, intensifying data management needs. Increasing regulatory emphasis on risk management frameworks, such as the FDA’s guidance updates and EMA’s recommendations post-2023, has compelled sponsors to integrate RBM to maintain compliance. Another key driver is the advancement of cloud-based monitoring platforms that enable real-time data analytics, reducing trial monitoring costs by up to 20%, as observed in recent phase II studies. Growing investments in decentralized clinical trials (DCTs) due to COVID-19 digital transformation trends have additionally expanded RBM adoption, especially in emerging regions focusing on telemedicine infrastructure.
Risk-Based Monitoring Market Development
In April 2024, Oracle Health launched a cloud-based Risk-Based Monitoring (RBM) platform integrated with AI capabilities, designed to improve the accuracy, efficiency, and scalability of clinical trial monitoring. The solution enables real-time risk detection, centralized oversight, and automated analytics, helping sponsors and CROs reduce operational costs while enhancing trial quality and regulatory compliance.
In 2022, TCS introduced an AI/ML-powered Risk-Based Monitoring solution as part of its TCS ADD™ clinical analytics platform, aimed at transforming how clinical trials are monitored. The solution allows proactive identification of study- and site-level risks through advanced data analytics, supporting smarter decision-making, improved patient safety, and optimized trial performance.
Key Players
Leading Companies of the Market
Syneos Health
PRA Health Sciences
Pharmaceutical Product Development (PPD)
PharmaLex GmbH
eClinical Solutions
BenevolentAI
Capgemini
Indegene
Saama Technologies
Medable Inc.
Several market players have adopted competitive strategies such as strategic partnerships with AI firms to bolster analytics capabilities and integration of RBM modules into broader eClinical suites. For example, Veeva Systems expanded its cloud-based RBM functionalities in late 2024, resulting in a 15% increase in client acquisition within six months. Similarly, Medidata’s acquisition of AI startup Syneos enhanced predictive risk scoring, facilitating faster regulatory compliance. These initiatives have significantly influenced market growth strategies and helped capture higher market shares.
Risk-Based Monitoring Market Future Outlook
Future growth will be driven by increasing clinical trial complexity and the shift toward decentralized and hybrid trial models. Integration of artificial intelligence, real-time analytics, and automation will further enhance monitoring efficiency. Regulatory support and cost optimization efforts will encourage broader adoption across pharmaceutical and biotech companies. As digital transformation continues in life sciences, risk-based monitoring will become an industry standard.
Risk-Based Monitoring Market Historical Analysis
The risk-based monitoring market developed as pharmaceutical companies sought alternatives to traditional, resource-intensive clinical trial monitoring methods. Initially, regulatory uncertainty limited adoption, but advancements in data analytics and centralized monitoring technologies improved acceptance. Over time, regulators increasingly supported risk-based approaches, recognizing their potential to improve trial efficiency and data quality. Adoption accelerated as clinical trials became more complex and globalized, reinforcing the market’s growth trajectory.
Sources
Primary Research Interviews:
Clinical Trial Managers
Regulatory Affairs Specialists
CRO Executives
Data Scientists
Quality Assurance Heads
Databases:
GlobalData Clinical Trials
Statista Life Sciences
FDA Regulatory Data
EMA Clinical Research Reports
Magazines:
Applied Clinical Trials
Pharmaceutical Technology
BioPharma Dive
Clinical Leader
Outsourcing-Pharma
Journals:
Clinical Trials Journal
Drug Information Journal
Therapeutic Innovation & Regulatory Science
Journal of Clinical Research
Contemporary Clinical Trials
Associations:
Drug Information Association
Association of Clinical Research Professionals
FDA,
European Medicines Agency
WHO
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients