Global factor VIII deficiency treatment Market is estimated to be valued at USD 2,783.6 Mn in 2025 and is expected to reach USD 3,943.0 Mn in 2032, exhibiting a compound annual growth rate (CAGR) of 5.1% from 2025 to 2032 Increase in product launches by key market players is expected to drive market growth over the forecast period. Moreover, an increase in the prevalence of hemophilia is also expected to drive market growth.
Analysts’ Views on Global Factor VIII Deficiency Treatment Market:
The Global factor VIII deficiency treatment market growth is driven by an increase in approvals by various regulatory authorities such as U.S. FDA. For instance, on February 23, 2023, Sanofi, a France-based multinational pharmaceutical and healthcare company, announced that they have received U.S. FDA approval for its efanesoctocog alfa, a first-in-class, high-sustained factor VIII replacement therapy, useful for the treatment of hemophilia A in adults
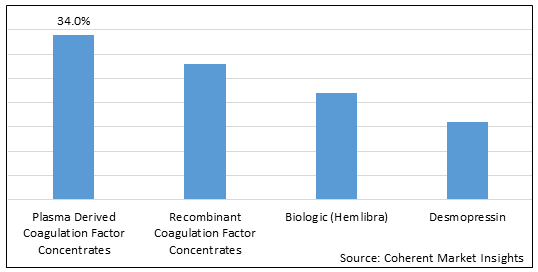
Figure 1. Global Factor VIII Deficiency Treatment Market Share (%), by Product Type, 2025
To learn more about this report, Download Free Sample
Global Factor VIII Deficiency Treatment Market- Driver
Increase in the research and development activities by key market players
Increase in the research and development activities by key market players for the treatment of hemophilia is expected to drive market growth over the forecast period. For instance, in December 2022, Pfizer Inc., a U.S.-based multinational biopharmaceutical company, announced positive top-line results from the Phase 3 BENEGENE-2 study evaluating fidanacogene elaparvovec, an investigational gene therapy, for the treatment of adult males with moderately severe to severe hemophilia B.
An increase in funding by non-government organizations
Increase in the initiatives by various government and non-government organizations for providing funding for hemophilia research and to develop novel treatment solutions is expected to propel the Global factor VIII deficiency treatment market growth for the forecast period. For instance, in September 2022, National Hemophilia Foundation, a U.S.-based non-government organization, announced that they have launched Pathway to Cures (P2C), a new venture philanthropy investment fund, under which the organization grants US$ 3.5 million in funding for research on blood-related disorders
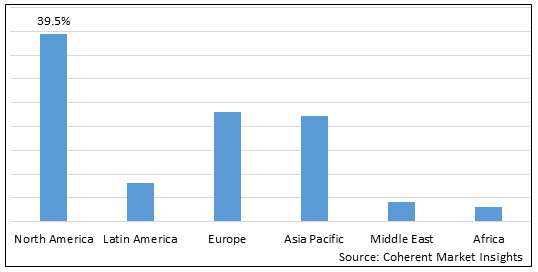
Figure 2. Global Factor VIII Deficiency Treatment Market Share (%), by Region, 2025
To learn more about this report, Download Free Sample
Global Factor VIII Deficiency Treatment Market- Regional Analysis
Among all regions, North America is expected to dominate the global market over the forecast period. This is attributed to North America holding a 39.5% market share and due to the presence of major players such as Baxter, Biogen, Inc., Bayer AG, and others contributing to the development of the factor VIII deficiency treatment market in North America.
The Asia Pacific region is expected to be the second dominating region over the forecast period, due to an increase in the number of people suffering from hemophilia. For instance, according to an article published in springer nature, an open-access scientific journal, in September 2020, the number of people suffering from hemophilia each year in India is between 100,000 – 125,000
Global Factor VIII Deficiency Treatment Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization declared it a public health emergency on January 30, 2020.
COVID-19 has affected the economy in three main ways: by directly affecting the production and demand for drugs and vaccines, by creating disruptions in distribution channels, and through its financial impact on firms and financial markets. Due to nationwide lockdowns, several countries such as China, India, Saudi Arabia, UAE, Egypt, and others faced problems with regard to the transportation of drugs and vaccines from one place to another.
Quarantine, traveling constraints, and social distancing measures are likely to lead to a steep decline in business and consumer spending. Furthermore, healthcare providers were facing challenges in terms of additional manpower, equipment, consumables, and other resources, which were required to ensure safety in hospitals and provide treatment to patients with other diseases. This has impacted the overall healthcare market negatively. Additionally, a decline in the number of patients visiting hospitals decreased, which is expected to affect the Global factor VIII deficiency treatment market over the forecast period.
Global Factor VIII Deficiency Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2,783.6 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.1% | 2032 Value Projection: | USD 3,943.0 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Baxter, Biogen, Inc., Bayer AG, CSL Behring, Ferring B.V., F. Hoffmann-La Roche AG, Pfizer, Inc., Kedrion, and Novo Nordisk A/S |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Factor VIII Deficiency Treatment Market Segmentation:
The Global factor VIII deficiency treatment market report is segmented into product type, and distribution channel
Based on product type, the market is segmented into plasma derived coagulation factor concentrates, recombinant coagulation factor concentrates, biologic (hemlibra), and desmopressin. Out of which, the recombinant coagulation factor concentrates segment is expected to dominate the Global factor VIII deficiency treatment market during the forecast period and this is attributed to an increase in the product launches by key market players.
Based on distribution channel, the market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. Out of which, the hospital segment is expected to dominate the market over the forecast period and this is attributed to an increase in the number of hospitals globally.
Among all segmentation, the product type segment has the highest potential due to an increase in the number of product approvals. For instance, Chugai Pharmaceutical Co., Ltd., a Japan-based pharmaceutical company, announced that it obtained regulatory approval from the Ministry of Health, Labour and Welfare for the anti-coagulation factor IXa/X humanized bispecific monoclonal antibody/coagulation factor VIII substitute Hemlibra [generic name: emicizumab (genetical recombination)] for patients with acquired hemophilia A.
Global Factor VIII Deficiency Treatment Market Cross Sectional Analysis:
In the product type segment, Biologic segment held a dominant position in the North America region due to an increase in the inorganic strategies between key market players. For instance, in June 2020, CSL Behring, a U.S.-based biopharmaceutical company, announced that it had acquired exclusive global license rights to commercialize AMT-061 (etranacogene dezaparvovec), a biologic based drug candidate indicated to treat hemophilia from Uniqure NV, a Netherlands based pharmaceutical company
Global Factor VIII Deficiency Treatment Market: Key Developments
In November 2022, the U.S. Food and Drug Administration announced that they have approved Hemgenix (etranacogene dezaparvovec), an adeno-associated virus vector-based gene therapy for the treatment of adults with Hemophilia A and B (congenital Factor IX and/or VIII deficiency)
In December 2021, Pfizer Inc., a U.S.-based multinational biopharmaceutical company, and Sangamo Therapeutics, Inc., a genomic medicines company, announced that their drug candidate giroctocogene fitelparvovec an investigational gene therapy for patients with moderately severe to severe hemophilia A, meet the regulatory requirements.
Global Factor VIII Deficiency Treatment Market: Restraint
High cost associated with hemophilia treatment
The major factors that can hamper the growth of the Global factor VIII deficiency treatment market over the forecast period include the high cost of hemophilia treatment. For instance, according to National Hemophilia Foundation, in May 2021, the average 6-month cost of hemophilia treatment is around US$ 300,000.
Global Factor VIII Deficiency Treatment Market: Key Players
Major players operating in the Global Factor VIII Deficiency Treatment market include Baxter, Biogen, Inc., Bayer AG, CSL Behring, Ferring B.V., F. Hoffmann-La Roche AG, Pfizer, Inc., Kedrion, and Novo Nordisk A/S
*Definition: Hemophilia is usually an inherited bleeding disorder in which the blood does not clot properly. This can lead to spontaneous bleeding as well as bleeding following injuries or surgery. Blood contains many proteins called clotting factors that can help to stop bleeding.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients