Respiratory Trainer is categorized under two categories based on product type, including resistance training devices and endurance training devices. Resistance training is the practice of breathing through a respiratory mouth-device, which is designed to constrain the airflow to the user, increase airway resistance, and consequently increase the work required for inhalation and exhalation by the respiratory muscles. Respiratory endurance is the ability of the human body to perform prolonged, moderate to higher intensity exercise. It is an important part of an individual's overall health. The heart and lungs absorb and transport more amount of oxygen during longer periods of physical activity. The term endurance is also known as respiratory endurance. It is an indicator of a person's physical potential and strength.
The global respiratory trainer market is estimated to be valued at US$ 481.83 million in 2022 and expected to reach US$ 844.72 million by 2030, witnessing a CAGR of 7.3% over the forecast period (2022-2030).
Figure 1. Global Respiratory Trainer Market Share (%), By Region, 2022
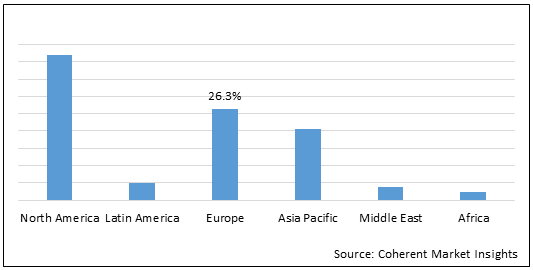
To learn more about this report, Download Free Sample
Increasing incidence of respiratory disorders is expected to drive growth of the global respiratory trainer market.
Increasing incidence of respiratory disorders such as asthma, chronic obstructive, and others is expected to drive demand for respiratory trainer devices for the treatment of these diseases. This is further expected to drive the global respiratory trainer market growth over the forecast period.
For instance, according to the Asthma and Allergy Foundation of America, in 2021, 25 million Americans had asthma. This equals to about 1 in 13 Americans, including 8% of adults and 7% of children, and about 20 million adults in the U.S., aged 18 years and older have asthma.
Respiratory Trainer Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 481.83 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 7.3% | 2030 Value Projection: | US$ 844.72 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Medline Industries, Inc., Koninklijke Philips N.V., Smiths Medical, Inc., Vyaire Medical, Inc., IngMar Medical, POWERbreathe International Limited, PN Medical, Aleas Europe LLC, Aspire Products, LLC, Airofit, Project Electronics Limited, Biegler GmbH, Nidek Medical India, Besmed Health Business Corp, Forumed S.L., and Angiplast Private Limited. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Figure 2. Global Respiratory Trainer Market Share (%) Analysis, By Product Type, 2022
To learn more about this report, Download Free Sample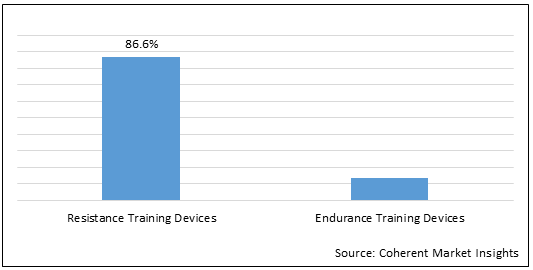
Increasing respiratory trainer device launches and approvals is expected to drive growth of the global respiratory trainer market.
Key players operating in market are focusing on launching respiratory trainer products. This is expected to propel growth of the global respiratory trainer market over the forecast period.
For instance, in September 1, 2021, Oumua, a manufacturer of breathing trainer, launched two versions of its breathing exercise device: Oumua Pro and Oumua AI. Oumua Pro features six resistance modes for inspiratory and expiratory muscle training, and Oumua AI adds smartphone connectivity, app-tracked progress, and artificial intelligence powered exercising guidance depending on the user’s performance and goals. Moreover, on September 21, 2021, the company launched a kickstarter campaign in order to increase awareness of the product as well as provide training to patients. The pre-order list of the project includes more than 10,000 confirmed reservations.
Global Respiratory Trainer Market – Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 outbreak in December 2019, the disease has spread to over 100 countries across the globe. The coronavirus (COVID 19) pandemic and consequent lockdown in various countries across the globe have impacted the financial status of businesses in all sectors. Supply chain and manufacturing activities have been disrupted globally, due to lockdowns implemented by governments, restricted movement, and other COVID-19 safety precautions. This has impacted the global respiratory trainer market negatively.
However, as the prevalence of respiratory diseases increases, the demand for respiratory trainers will increase, which is expected to drive the global respiratory trainer market growth over the forecast period
Moreover, key players are focusing on launching respiratory trainer devices to reduce breathing discomfort by reducing the effort required to breathe. For instance, in August 2021, IngMar Medical, a manufacturer and distributor of respiratory trainer devices, announced the launch of RespiPro, IngMar Medical's next-generation breathing and ventilation training solution. The solution includes realistic breathing simulator, the ASL 5000,user-friendly software, realistic patient monitors, and a compact intensive care unit breathing mannequin.
Global Respiratory Trainer Market: Restraint
Product recall and critical regulatory compliance procedures may act as a restraint for growth of the respiratory trainer market. For instance, in July 2021, the U.S. Food and Drug Administration (FDA) directed Philips Respironics, a medical devices company to recall millions of sleep and respiratory devices following concerns that foam in the devices, which is used to reduce sound and vibration, may break into particles and enter the air hose of the device and be inhaled by the user.
Moreover, in 2020, the U.S. FDA issued guidance to formulate a policy to help expand the availability of ventilators as well as other respiratory devices and their accessories during the COVID-19 pandemic.
Key Players
Major players operating in the global respiratory trainer market include Medline Industries, Inc., Koninklijke Philips N.V., Smiths Medical, Inc., Vyaire Medical, Inc., IngMar Medical, POWERbreathe International Limited, PN Medical, Aleas Europe LLC, Aspire Products, LLC, Airofit, Project Electronics Limited, Biegler GmbH, Nidek Medical India, Besmed Health Business Corp, Forumed S.L., and Angiplast Private Limited.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients