Respiratory Syncytial Virus Diagnostics Market Size and Forecast – 2025 – 2032
The Global Respiratory Syncytial Virus Diagnostics Market size is estimated to be valued at USD 1.5 billion in 2025 and is expected to reach USD 3.2 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 11.2% from 2025 to 2032.
Global Respiratory Syncytial Virus Diagnostics Market Overview
RSV diagnostic products are designed to detect the presence of respiratory syncytial virus through molecular, antigen, or serological testing. These include rapid antigen detection kits for point-of-care use, RT-PCR assays for high-sensitivity detection, and multiplex panels that identify RSV along with other respiratory pathogens. Newer diagnostic systems feature automated platforms and digital readouts to improve accuracy and speed, allowing clinicians to make timely treatment decisions. Compact, cartridge-based devices and at-home test kits have made RSV testing more accessible, particularly during peak viral seasons.
Key Takeaways
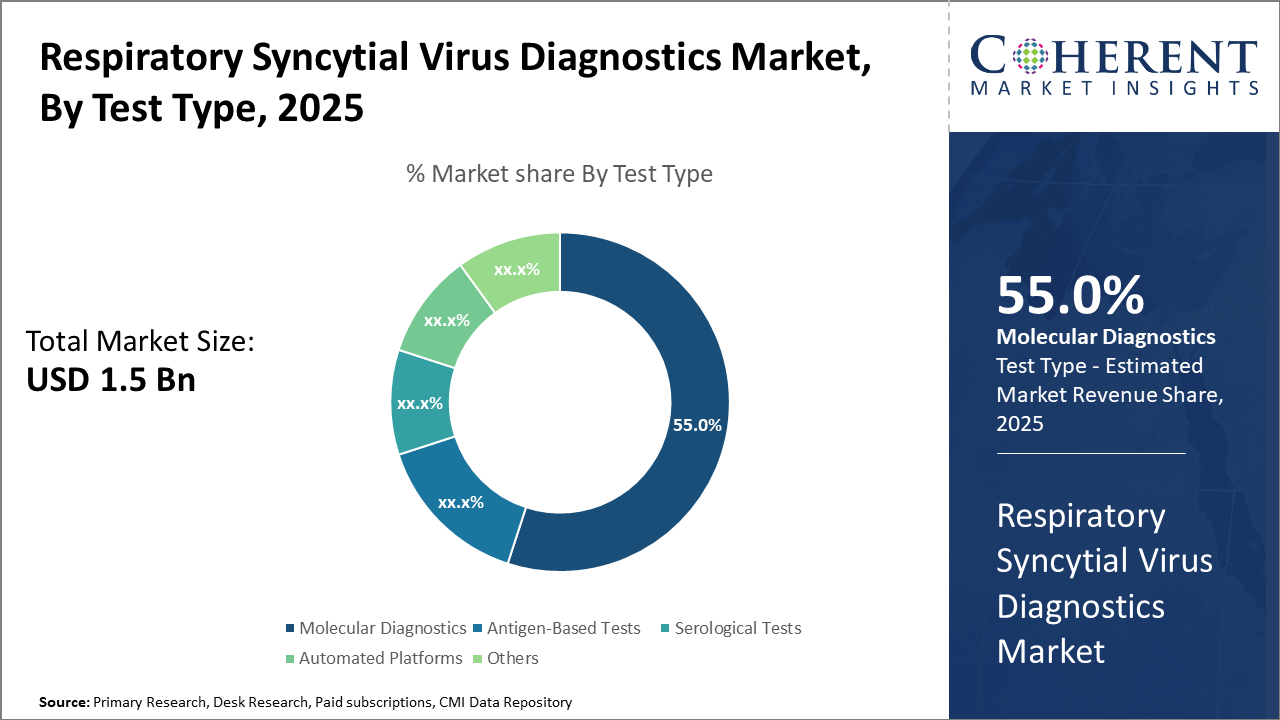
The Molecular Diagnostics segment dominates the test type category, showing over 55% market share, driven by high specificity and rapid turnaround times.
Hospitals and Clinics remain the primary end user, accounting for nearly 60% of market revenue, emphasizing institutional testing demand.
The Pediatric Care application demonstrates the dominant share, fueled by rising RSV hospitalizations in children under five years, increasing by over 20% in 2024.
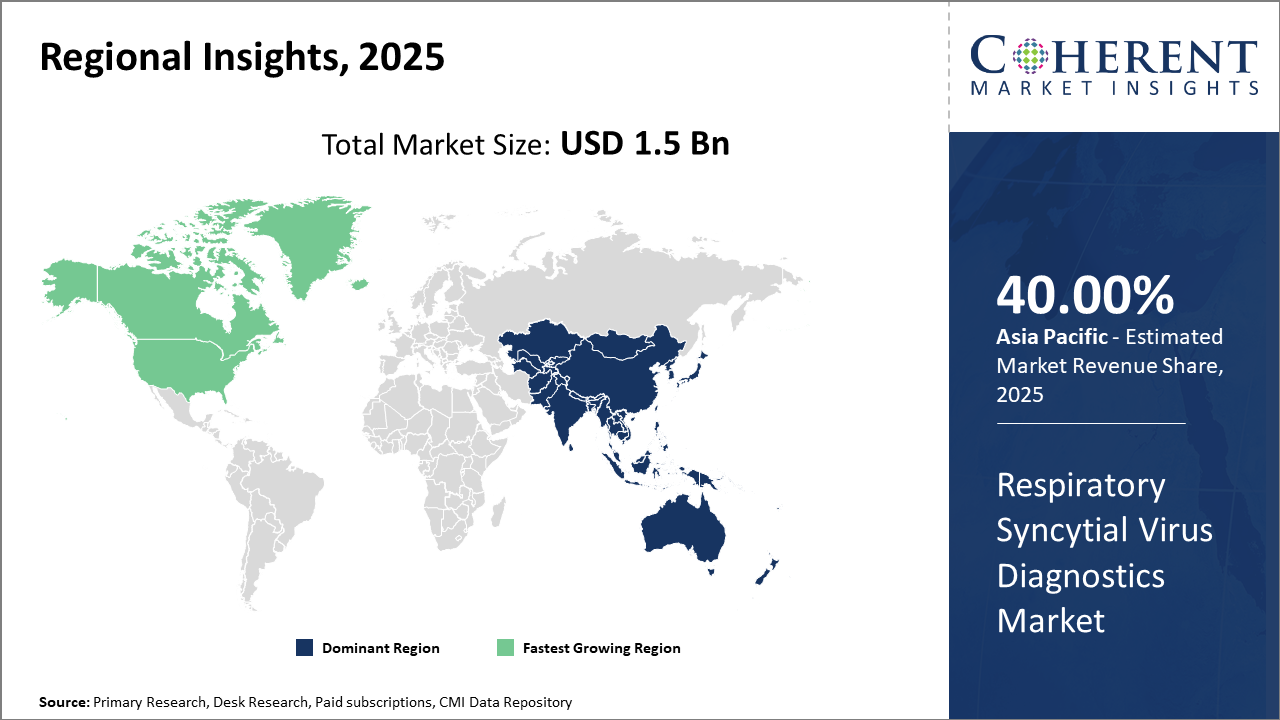
North America leads the regional market share with approximately 40%, supported by strong healthcare infrastructure and reimbursement policies.
Asia Pacific emerges as the fastest-growing regional market, exhibiting a CAGR exceeding 13%, propelled by government initiatives and expanding diagnostic infrastructure.
Europe holds a stable market presence, with Germany and the U.K. contributing substantially through advanced diagnostic adoption and testing programs.
Respiratory Syncytial Virus Diagnostics Market Segmentation Analysis
To learn more about this report, Download Free Sample
Respiratory Syncytial Virus Diagnostics Market Insights, By Diagnostic Type
Molecular Diagnostics dominate the market share. Molecular Diagnostics lead due to their high sensitivity and specificity, and rapid turnaround times, making them the preferred choice in hospital laboratories and diagnostic centers. The fastest-growing subsegment is Automated Platforms, benefiting from automation trends that reduce manual errors and support high-throughput testing, gaining traction in 2024 with a 12% increase in adoption. Antigen-Based Tests maintain steady usage attributable to their rapid results and ease of use, suitable for point-of-care settings. Serological Tests are generally used for epidemiological assessments, while the Others segment covers niche diagnostics and emerging technologies.
Respiratory Syncytial Virus Diagnostics Market Insights, By End-User
Hospitals and Clinics dominate the market share due to their role as primary care facilities for RSV-infected patients and routine screening protocols. Diagnostic Laboratories are experiencing fast growth propelled by outsourcing trends and centralized testing services offering multiplexing capabilities. Point-of-Care Testing Centers are expanding, fueled by the demand for rapid and decentralized diagnostics, especially in community and rural settings. Research Institutes contribute through innovation and validation of novel diagnostic techniques. The Others segment includes specialty settings like neonatal care units.
Respiratory Syncytial Virus Diagnostics Market Insights, By Application
Pediatric Care holds the largest market share, reflecting the high prevalence of RSV infections in children under five, driving diagnostic testing protocols in clinical practice. Neonatal Screening is the fastest-growing subsegment due to rising preterm birth rates and increased awareness of RSV risk in neonates. Geriatric Care is gaining traction with an aging global population susceptible to severe RSV complications. Community Health Programs incorporate widespread surveillance and early intervention initiatives, enhancing market scope. Others include workplace screening and immunocompromised patient monitoring.
Respiratory Syncytial Virus Diagnostics Market Trends
The Respiratory Syncytial Virus Diagnostics Market is observing significant momentum from the integration of multiplex testing platforms that combine RSV diagnosis with other respiratory pathogens, streamlining clinical decision-making processes.
For example, in 2024, a prominent diagnostic company launched a multiplex respiratory panel that increased patient throughput by 25% in Europe.
Additionally, AI-driven diagnostic tools are gaining traction, offering predictive insights into RSV seasonal trends, which aid healthcare providers in proactive outbreak management.
Home-based diagnostic kits, propelled by telemedicine growth post-pandemic, are facilitating early RSV detection, enhancing patient outcomes by reducing hospital visits.
These technological advancements have created a market shift towards more precise, rapid, and accessible testing costs.
Respiratory Syncytial Virus Diagnostics Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Respiratory Syncytial Virus Diagnostics Market Analysis and Trends
In North America, the dominance in the Respiratory Syncytial Virus Diagnostics Market is reinforced by a robust healthcare infrastructure, high healthcare spending, and supportive reimbursement policies for RSV testing. The U.S. accounts for the largest market share, approximately 40%, bolstered by high RSV hospitalization rates and the adoption of innovative diagnostic modalities. Major players have established significant manufacturing bases and clinical partnerships here, enhancing market revenue.
Asia Pacific Respiratory Syncytial Virus Diagnostics Market Analysis and Trends
Meanwhile, the Asia Pacific region exhibits the fastest growth with a CAGR exceeding 13%. This acceleration is driven by increased government focus on infectious disease surveillance, expansion of diagnostic facilities, and rising awareness of RSV health risks. Countries like India, China, and Australia are investing heavily in RSV screening programs. Market companies are partnering with local healthcare providers to improve testing accessibility, fueling performance in this region.
Respiratory Syncytial Virus Diagnostics Market Outlook for Key Countries
USA Respiratory Syncytial Virus Diagnostics Market Analysis and Trends
The USA's market remains a cornerstone of the Respiratory Syncytial Virus Diagnostics Market owing to its advanced diagnostic infrastructure and significant RSV-related healthcare burden. In 2024, U.S. hospitals reported a 15% increase in RSV diagnostic tests, influenced by expanding multiplex platforms and increased demand in pediatric and geriatric care units. Key players like Abbott and Roche have rolled out rapid PCR-based diagnostic tools and expanded point-of-care offerings, contributing notably to overall market revenue and maintaining high market share.
India Respiratory Syncytial Virus Diagnostics Market Analysis and Trends
India's Respiratory Syncytial Virus Diagnostics Market is rapidly expanding due to government initiatives targeting childhood respiratory infections and growing awareness about RSV testing. Recent health campaigns and integration of diagnostics into national immunization programs in 2025 have led to a 20% increase in RSV testing across public healthcare facilities. Market companies are collaborating with local diagnostic labs to introduce cost-effective antigen-based and molecular tests, increasing accessibility in rural and urban demographics. This momentum places India as a critical growth hub within the Asia Pacific region.
Analyst Opinion
The rising adoption of molecular diagnostic techniques, particularly PCR-based assays, stands as a critical demand-side indicator driving the market share significantly. In 2024, molecular diagnostics accounted for over 55% of the total market revenue, propelled by their superior sensitivity and specificity compared to traditional antigen-based tests. For instance, the uptake of real-time PCR assays in clinical virology labs grew by 18% between 2023 and 2024, reflecting the market’s shift towards these methods.
Supply-side dynamics reveal an expansion in production capacity of diagnostic kits, with multiple manufacturers scaling up production lines to meet surging RSV testing demands, especially evident during recent winter outbreaks in North America and Europe. In 2025 Q1, production volume rose by 22% compared to the previous year, contributing to the overall market growth rate.
Pricing strategies are evolving as new entrants leverage cost-effective biosensor technologies, challenging the traditional market players and expanding affordability. Market research shows a 9% average price decline in rapid diagnostic tests in 2024, encouraging wider adoption in emerging economies, thereby broadening market penetration.
Market players’ emphasis on diversified use cases, including prenatal diagnostics and multiplex panel testing for respiratory pathogens, has created new demand avenues. The use of RSV testing in pediatric hospital settings surged by 30% in 2024, reflecting increased screening protocols leading to faster diagnosis and improved patient management.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 1.5 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 11.2% | 2032 Value Projection: | USD 3.2 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Abbott Laboratories, Roche Diagnostics, Thermo Fisher Scientific, BioMérieux, Quidel Corporation, Hologic Inc., Siemens Healthineers, Fujirebio, LumiraDx, Meridian Bioscience, QIAGEN. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Respiratory Syncytial Virus Diagnostics Market Growth Factors
The rise in RSV-related hospitalizations, particularly in pediatric and elderly demographics, is driving market revenue growth significantly. WHO reported a 25% increase in RSV-associated respiratory illnesses globally in 2024, underscoring diagnostic market demand. Increasing government and private sector funding in infectious disease surveillance has accelerated market growth in the Asia Pacific, with countries like India launching national RSV screening programs in 2025.
The trend towards multiplex respiratory pathogen testing, integrating RSV diagnostics with influenza and COVID-19 panels, is fostering higher adoption rates, with multiplex tests accounting for 30% of total RSV diagnostic revenues in 2024. Lastly, advancements in point-of-care diagnostics enabling rapid, decentralized testing are broadening access in resource-limited settings, affecting overall market dynamics positively.
Respiratory Syncytial Virus Diagnostics Market Development
In May 2025, Sekisui Diagnostics introduced the OSOM® RSV Test, a rapid immunochromatographic assay cleared for point-of-care use and CLIA-waived, designed for children aged six months to six years and adults aged 60 and older. The test uses an anterior nasal swab specimen and delivers results in approximately 15 minutes, supporting non-invasive, rapid detection of Respiratory Syncytial Virus (RSV) and targets high-risk patient segments with an easy and cost-efficient workflow.
In September 2024, Roche Diagnostics launched the cobas® Respiratory flex Test, the first diagnostic test to utilize its proprietary TAGS (Temperature-Activated Generation of Signal) technology. This high-throughput multiplex PCR assay enables simultaneous detection of up to 12 common respiratory viruses — including influenza A/B, RSV, adenovirus, human metapneumovirus, enterovirus/rhinovirus, parainfluenza 1–4 and coronavirus strains — in a single patient sample on the cobas® 5800/6800/8800 systems. The system allows laboratories to tailor pathogen testing by season, patient demographic or setting, improving diagnostic speed and resource-use efficiency.
Key Players
Leading Companies of the Market
Abbott Laboratories
Roche Diagnostics
Thermo Fisher Scientific
BioMérieux
Quidel Corporation
Hologic Inc.
Siemens Healthineers
Fujirebio
LumiraDx
Meridian Bioscience
QIAGEN
Competitive strategies feature technological innovation, such as Abbott’s launch of near-patient molecular testing platforms, enhancing rapid diagnosis capabilities with turnaround times under an hour, which increased their market footprint in North America by approximately 15% in 2024. Roche Diagnostics expanded its manufacturing capacity in Europe during 2024, resulting in a 20% rise in supply and enabling penetration into underdeveloped regions, demonstrating the impact of capacity scalability on market share expansion.
Respiratory Syncytial Virus Diagnostics Market Future Outlook
The future of RSV diagnostics lies in high-sensitivity molecular platforms, digital reporting integration, and at-home testing capabilities. AI-based interpretation and smartphone-linked diagnostic readers will enhance clinical decision-making and outbreak surveillance. Multiplex systems capable of identifying RSV, influenza, and emerging respiratory viruses from a single sample will dominate hospital workflows. As preventive monoclonal antibodies and vaccines gain widespread adoption, real-time diagnostic monitoring will play a key role in disease control programs. Accessibility, automation, and affordability will define the next evolution of RSV diagnostic products.
Respiratory Syncytial Virus Diagnostics Market Historical Analysis
RSV testing initially relied on culture-based and immunofluorescence assays, which were time-consuming and required specialized laboratory infrastructure. The advent of rapid antigen detection tests in the late 1990s provided clinicians with faster, point-of-care diagnostic options. The introduction of molecular diagnostic technologies, particularly RT-PCR and multiplex panels, revolutionized accuracy and sensitivity during the 2010s. This shift was accelerated by the COVID-19 pandemic, which emphasized the need for simultaneous respiratory pathogen testing and rapid turnaround. Automation and miniaturization further expanded testing capacity across hospitals, clinics, and home settings.
Sources
Primary Research Interviews:
Pulmonologists
Virologists
Diagnostic Laboratory Directors
Pediatric Infectious Disease Specialists
Databases:
WHO Global Health Observatory
CDC Respiratory Disease Data
GlobalData Diagnostics Reports
Magazines:
Diagnostics World
Medical Device Network
Clinical Lab Products
MedTech Insight
Journals:
Journal of Clinical Microbiology
Diagnostic Microbiology and Infectious Disease
Newspapers:
The Washington Post (Health)
The Guardian (Science)
The Hindu (Health)
Associations:
World Health Organization (WHO)
Infectious Diseases Society of America (IDSA)
American Thoracic Society (ATS)
European Society of Clinical Microbiology and Infectious Diseases (ESCMID)
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients