Respiratory Distress Syndrome Treatment Market Size and Forecast – 2025 – 2032
The Global Respiratory Distress Syndrome Treatment Market size is estimated to be valued at USD 4.75 billion in 2025 and is expected to reach USD 8.60 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 8.5% from 2025 to 2032.
Global Respiratory Distress Syndrome Treatment Market Overview
Products in the respiratory distress syndrome treatment market include therapeutic drugs, respiratory support devices, and clinical care solutions used to manage impaired lung function. Key products include surfactant replacement therapies, oxygen delivery systems, ventilators, and continuous positive airway pressure devices. These treatments aim to improve oxygen exchange and lung compliance in patients with compromised respiratory systems. They are primarily used in neonatal intensive care units and critical care settings.
Key Takeaways
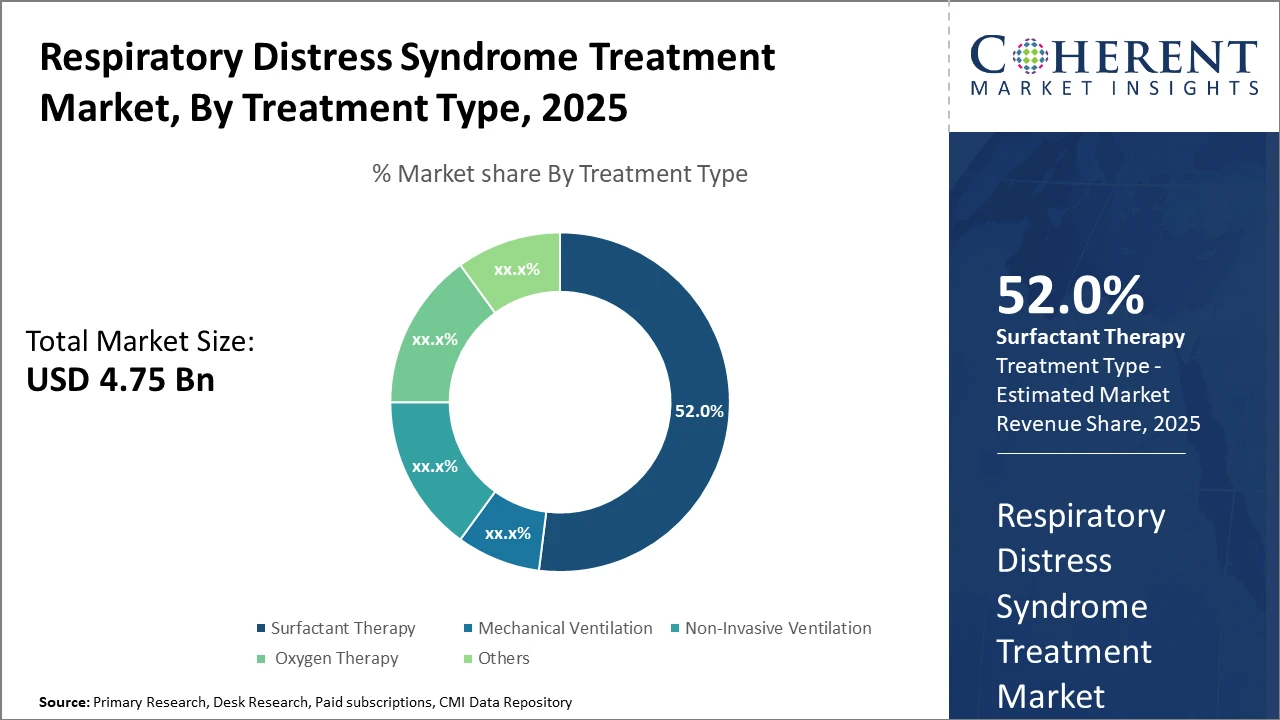
The Surfactant Therapy segment dominates, commanding over 52% of the market share, owing to its proven clinical benefits and broad application across neonatal and adult cases.
Mechanical Ventilation is recognized as the fastest-growing subsegment, fueled by escalating ICU cases globally demanding advanced life support.
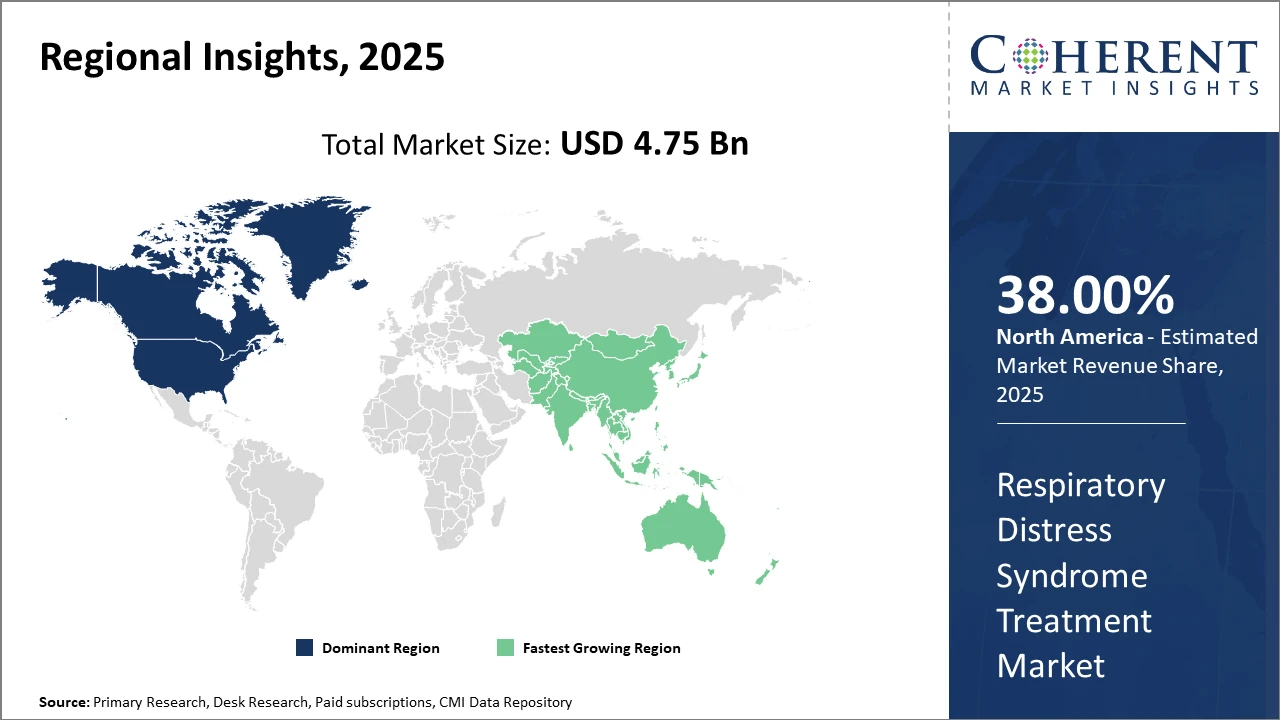
Within regional markets, North America holds the largest share, attributed to robust healthcare infrastructure, higher healthcare expenditure, and the strong presence of leading market companies.
Asia Pacific emerges as the fastest-growing region, supported by an expanding patient base, increasing healthcare investments, and growing government initiatives to improve neonatal care.
Respiratory Distress Syndrome Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
Respiratory Distress Syndrome Treatment Market Insights, By Treatment Type
Surfactant Therapy dominates the market share. Surfactant Therapy's dominance is attributable to its established clinical efficacy in reducing mortality and morbidity in neonatal respiratory distress, making it the cornerstone of treatment worldwide. The growing preference for advanced synthetic surfactants due to their improved pharmacological profiles further solidifies their position. Meanwhile, Non-Invasive Ventilation is the fastest growing, propelled by clinical evidence supporting reduced pulmonary injuries and hospital stay, encouraging its adoption in both neonatal and adult care settings.
Respiratory Distress Syndrome Treatment Market Insights, By Patient Type
The Patient Type segment is divided into Neonatal, Adult, Pediatric, and Others, with Neonatal patients dominating market share. The prevalence of premature births and respiratory complications in newborns underpins this dominance. Enhanced neonatal care protocols and surfactant availability have significantly expanded treatment penetration. Adult patients represent the fastest-growing subsegment, primarily due to increased incidences of adult respiratory distress syndrome triggered by chronic diseases and acute lung injuries. Pediatric patients constitute a smaller but steady market, with respiratory infections and congenital abnormalities driving demand.
Respiratory Distress Syndrome Treatment Market Insights, By Application
Hospital ICUs dominate the market share. The critical care environment in ICUs hosts the highest need for advanced respiratory treatment options, driven by complex cases in both neonates and adults. Neonatal Care Units represent the fastest-growing segment, emphasizing specialized respiratory management and early intervention, which have been boosted by increased government funding and healthcare policies focusing on pediatric health. Ambulatory Care and Home Healthcare are emerging areas benefiting from telemedicine and portable device innovations, contributing to market breadth but currently accounting for smaller shares relative to institutional settings.
Respiratory Distress Syndrome Treatment Market Trends
The Respiratory Distress Syndrome Treatment market is witnessing transformative trends driven by technological advances and demographic pressures.
The rise of non-invasive ventilation techniques coupled with surfactant therapy optimization marks a pivotal shift from traditional mechanical ventilation reliance.
For example, in 2025, hospitals in Europe reported a 22% increase in non-invasive ventilation as first-line treatment, reducing complications and length of hospital stays.
Furthermore, personalized and data-driven treatment protocols are gaining traction, improving outcomes and guiding market evolution.
Respiratory Distress Syndrome Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Respiratory Distress Syndrome Treatment Market Analysis and Trends
Regionally, North America is the dominating region, holding over 38% of the Respiratory Distress Syndrome Treatment market share. This is driven by high healthcare spending, leading hospital networks, and active contributions from market players such as Pfizer and Novartis.
Asia Pacific Respiratory Distress Syndrome Treatment Market Analysis and Trends
Conversely, Asia Pacific represents the fastest-growing region, exhibiting a CAGR of approximately 10.4%, bolstered by increasing government healthcare budgets, rising awareness of neonatal health issues, and rapid adoption of novel medical technologies. The presence of emerging players in China and India, along with expanding neonatal care infrastructure, accelerates this regional growth.
Respiratory Distress Syndrome Treatment Market Outlook for Key Countries
USA Respiratory Distress Syndrome Treatment Market Analysis and Trends
The USA accounts for a significant portion of the market owing to advanced healthcare infrastructure, widespread adoption of cutting-edge respiratory therapies, and robust research investments. In 2024, the U.S. neonatal care units utilized surfactant therapy in over 76% of preterm infant respiratory distress cases, underscoring the adoption of best practices. Key market players, including Pfizer and Abbott, have invested heavily in R&D and strategic collaborations with major hospital networks, aligning with evolving treatment protocols.
India Respiratory Distress Syndrome Treatment Market Analysis and Trends
India's Respiratory Distress Syndrome Treatment market is rapidly expanding due to rising neonatal mortality concerns and improved healthcare accessibility. The government’s push for enhanced neonatal health outcomes through programs like the National Health Mission has led to increased availability of respiratory distress medications and devices in tertiary and secondary care centers. Market players have intensified focus on affordable synthetic surfactants, with companies like Chiesi Farmaceutici expanding manufacturing capacity in India.
Analyst Opinion
One actionable insight is the rising utilization of synthetic surfactants in treating neonatal respiratory distress syndrome, directly influencing market revenue. A recent clinical deployment from 2024 reported that synthetic surfactants accounted for over 42% of total surfactant therapy usage globally, supported by improved efficacy and cost-effectiveness relative to animal-derived alternatives.
Another crucial indicator is the expanding adoption of non-invasive ventilation methods complementing pharmacological treatment. Data from 2025 demonstrated a 15% year-over-year increase in hospitals implementing continuous positive airway pressure (CPAP) as a frontline treatment, driving demand for integrated respiratory distress syndrome treatment protocols.
Micro-level analysis reveals heightened import rates of respiratory support devices in emerging markets during 2024, with countries like India and Brazil witnessing a 28% surge. This demand surge reflects growing healthcare infrastructure investments and increased awareness of early intervention benefits in respiratory distress.
Supply chain improvements and increased manufacturing capacity for surfactants and respiratory aids have played a vital role. Industry production capacity increased by 12% in 2024 alone, with newly commissioned facilities in North America and the Asia Pacific providing a steady supply chain, thus enhancing the market share of manufacturers focusing on these regions.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 4.75 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.5% | 2032 Value Projection: | USD 8.60 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Vyaire Medical, Inc., GE Healthcare, Philips Healthcare, Smiths Medical, Hollister Incorporated, Masimo Corporation, Fresenius SE & Co. KGaA, Baxter International Inc., Cardinal Health, Inc., Medtronic plc. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Respiratory Distress Syndrome Treatment Market Growth Factors
The rise in premature births globally contributes directly to increased demand for neonatal
Respiratory distress syndrome treatment, with the World Health Organization reporting a 10% increment in preterm deliveries between 2023 and 2025. Technological advancements in neonatal care equipment and diagnostic capabilities have improved patient outcomes, spurring market revenue growth. Growing awareness and expanding reimbursement policies across developed and emerging economies facilitate access to high-cost treatment regimens, underpinning market expansion. Furthermore, the increasing incidence of adult respiratory distress syndrome due to chronic illnesses and ARDS from trauma victims further boosts the demand, with the U.S. alone witnessing a 23% rise in adult patient admissions requiring respiratory distress interventions in 2024.
Respiratory Distress Syndrome Treatment Market Development
In late 2024, Boehringer Ingelheim advanced its immunology portfolio with the launch of a novel inflammation modulator, targeting improved control of chronic inflammatory conditions. The therapy was designed to address unmet needs in immune-mediated diseases by offering enhanced efficacy with a differentiated mechanism of action, reinforcing Boehringer Ingelheim’s long-term focus on innovation in inflammation and autoimmune care.
In September 2024, Medtronic introduced the VitalFlow ECMO system, a next-generation extracorporeal membrane oxygenation platform developed to support patients with severe cardiac and respiratory failure. The system features improved flow control, ease of use, and enhanced patient monitoring, strengthening Medtronic’s leadership in critical care technologies and advanced life-support solutions.
Key Players
Leading Companies of the Market
Vyaire Medical, Inc.
GE Healthcare
Philips Healthcare
Smiths Medical
Hollister Incorporated
Masimo Corporation
Fresenius SE & Co. KGaA
Baxter International Inc.
Cardinal Health, Inc.
Medtronic plc
Several leading market players have adopted strategic alliances and acquisitions to bolster their product pipeline and regional presence. For instance, Pfizer’s recent acquisition of a biotech firm specializing in synthetic surfactants enhanced its product portfolio, resulting in a 20% uplift in market share during 2024 within North America. Similarly, Chiesi Farmaceutici executed a collaboration with leading hospitals in Europe to accelerate clinical trials of innovative respiratory therapies, propelling its business growth in that region by 14% last year.
Respiratory Distress Syndrome Treatment Market Future Outlook
The RDS treatment market’s future will be shaped by ongoing innovation in personalized respiratory support, integration of real-time monitoring systems, and development of novel pharmacotherapies that enhance gas exchange and reduce inflammation. Next-generation ventilation systems will incorporate machine learning algorithms to optimize settings and minimize lung trauma. Biologic therapies and regenerative approaches targeting alveolar repair may emerge as adjunct treatments. Growing emphasis on early detection and home-based respiratory support will expand care beyond intensive care units. As global populations age and respiratory conditions remain prevalent, healthcare investment in improved RDS diagnostics and therapies will sustain market growth.
Respiratory Distress Syndrome Treatment Market Historical Analysis
The respiratory distress syndrome (RDS) treatment market originally focused on supportive care for infants and adults with impaired lung function. In neonatology, surfactant replacement therapies became a breakthrough in the late 20th century, dramatically reducing morbidity and mortality in preterm infants by improving lung compliance. Mechanical ventilation and CPAP (continuous positive airway pressure) systems emerged as critical supportive technologies. Over time, advances in non-invasive ventilation, improved oxygen delivery systems, and better understanding of ventilator-associated lung injury transformed care protocols. In adult respiratory distress syndrome (ARDS), landmark clinical research redefined ventilation strategies and fluid management, emphasizing protective lung strategies. The expansion of critical care infrastructure and respiratory therapy specialties further supported the development of comprehensive treatment portfolios.
Sources
Primary Research Interviews:
Neonatologists
Pulmonologists
ICU Physicians
Respiratory Therapists
Medical Device Specialists
Databases:
WHO Health Statistics
CDC Respiratory Data
OECD Health Data
Magazines:
Respiratory Care
Medical Device Network
Healthcare Today
MedTech Insight
Clinical Care Journal
Journals:
American Journal of Respiratory Medicine
Pediatric Pulmonology, Critical Care Medicine
Respiratory Research Journal
Neonatology Journal
Newspapers:
Reuters Health
Financial Times (Healthcare)
The New York Times (Health)
The Guardian (Health)
The Hindu
Associations:
American Thoracic Society
European Respiratory Society
World Health Organization
Society of Critical Care Medicine
American Academy of Pediatrics
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients