Remicade Biosimilars Market Size and Forecast – 2025 – 2032
The Global Remicade Biosimilars Market size is estimated to be valued at USD 3.8 billion in 2025 and is expected to reach USD 9.1 billion by 2032, exhibiting a compound annual growth rate (CAGR) of 13.5% from 2025 to 2032.
Global Remicade Biosimilars Market Overview
Remicade biosimilars are monoclonal antibody-based biologic products designed to closely replicate infliximab, a TNF-α inhibitor originally marketed under the brand name Remicade. These biosimilars are used to treat autoimmune diseases such as rheumatoid arthritis, Crohn’s disease, ulcerative colitis, psoriasis, and ankylosing spondylitis. The product class includes approved biosimilars such as Inflectra, Renflexis, and Ixifi, which provide comparable efficacy, safety, and immunogenicity to the originator. Most formulations are supplied as lyophilized powders or prefilled vials for intravenous infusion. Product differentiation lies in manufacturing cell lines, purification processes, and formulation stability, ensuring consistent pharmacokinetics and therapeutic outcomes.
Key Takeaways
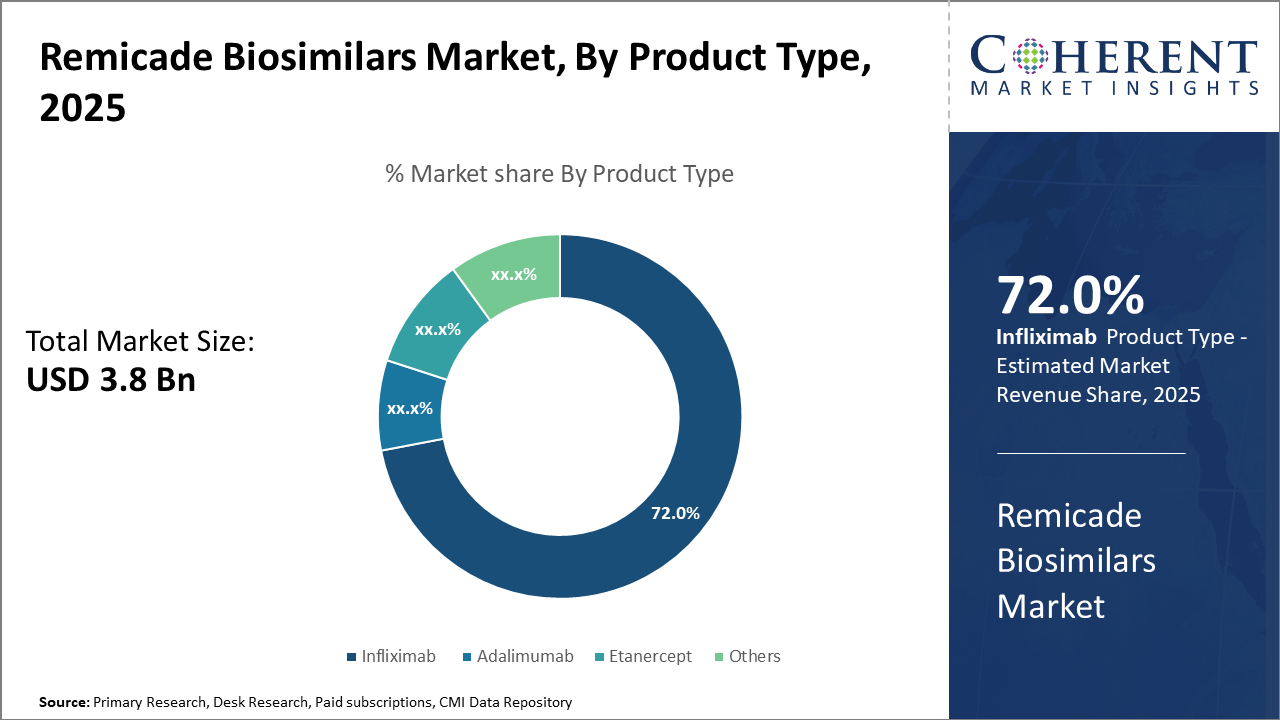
The infliximab segment dominates the Remicade Biosimilars market, holding over 72% industry share, driven by its extensive indication coverage and earlier market entry.
The adalimumab biosimilars subsegment is the fastest-growing, propelled by expanded approvals and demand in dermatology and rheumatology sectors.
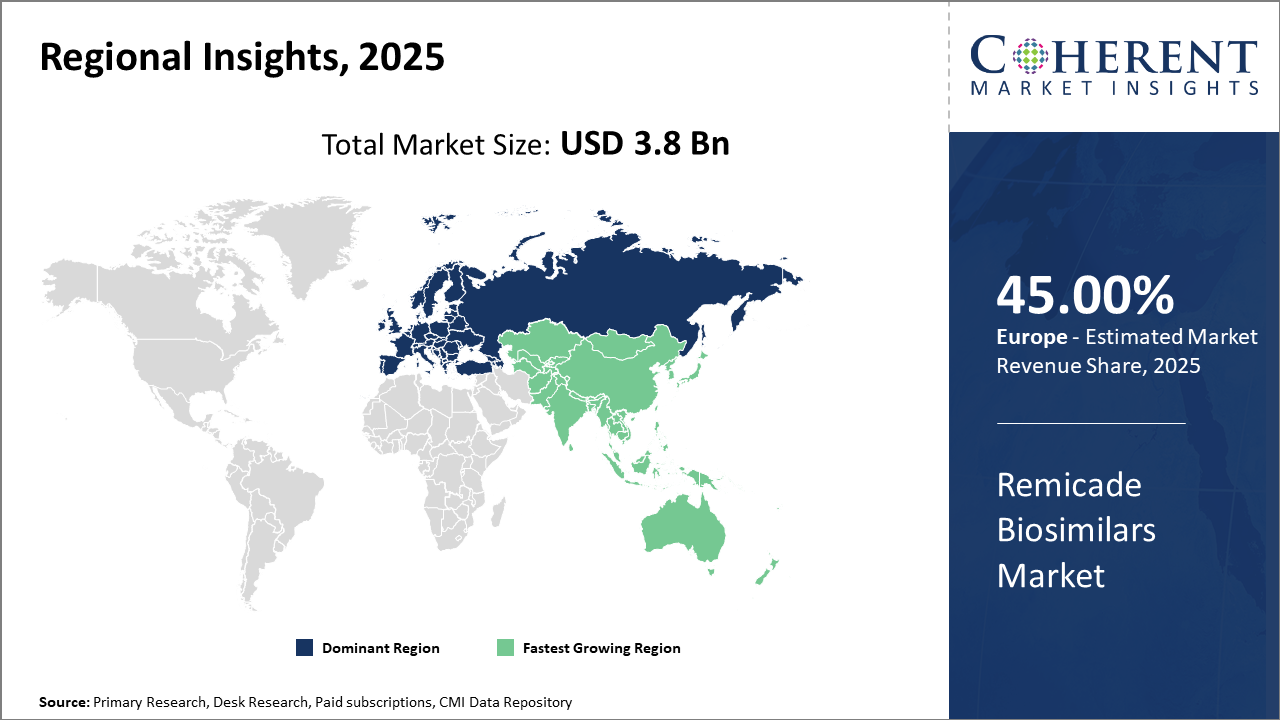
Regionally, Europe continues to dominate with a commanding 45% market share due to early biosimilar adoption and supportive regulatory ecosystems.
Meanwhile, the Asia Pacific is the fastest-growing region, exhibiting a CAGR surpassing 16% given expanding healthcare infrastructure and a rising patient base.
Remicade Biosimilars Market Segmentation Analysis
To learn more about this report, Download Free Sample
Remicade Biosimilars Market Insights, By Product Type
Infliximab biosimilars lead due to their early entry and wide indication coverage in treating rheumatoid arthritis, Crohn’s disease, and ulcerative colitis. Their robust clinical data and acceptance in major healthcare systems contribute significantly to their dominance. The fastest-growing subsegment, Adalimumab biosimilars, benefits from recent patent cliffs and expanding indications into dermatology and immunology, fueling market growth. Etanercept and others represent niche usage with growing but comparatively smaller adoption rates.
Remicade Biosimilars Market Insights, By Indication
Rheumatoid Arthritis represents the largest share due to its high prevalence globally and established biosimilar treatment protocols, resulting in significant market revenue contributions. Crohn’s disease is the fastest-growing indication, reflecting rising chronic inflammatory bowel disease cases worldwide and expanding off-label biosimilar usage. Psoriasis and Ulcerative Colitis have steady adoption rates, while other indications, such as ankylosing spondylitis, represent emerging niches.
Remicade Biosimilars Market Insights, By End User
Hospitals maintain dominance due to the administration of biologics within clinical settings and their billing infrastructure supporting reimbursement. Biosimilars in hospitals target critical patient populations with autoimmune diseases, benefiting from robust demand and strong hospital formulary inclusion. The fastest growing subsegment is Specialty Clinics, which increasingly cater to outpatient biologic administration and direct biosimilar prescription for diseases like psoriasis and Crohn’s disease. Retail Pharmacies remain supportive channels but are limited by prescription regulations and insurance coverage constraints.
Remicade Biosimilars Market Trends
Remicade Biosimilars market trends emphasize a rapid shift toward biosimilar interchangeability, facilitating substitution without prescriber consultation, especially in European countries such as the UK and Germany. This regulatory evolution accelerates uptake, thus elevating market revenue and competitive positioning.
Additionally, digital health integration supporting biosimilar treatment adherence is gaining traction, with platforms enabling remote monitoring that enhances patient outcomes in diseases like Crohn’s and rheumatoid arthritis.
Regionally, Europe dominates the market, accounting for approximately 45% of total market share, owing to early biosimilar adoption and streamlined regulatory approvals. The region benefits from established healthcare reimbursement models and a high disease burden, which drive demand.
Asia Pacific is the fastest-growing region with a CAGR exceeding 16%, propelled by increasing healthcare expenditure, expanding healthcare infrastructure, and rising patient populations in countries like India and China.
Furthermore, government initiatives promoting biosimilar use, coupled with local manufacturing capacity expansion, underpin this rapid growth.
Remicade Biosimilars Market Insights, By Geography
To learn more about this report, Download Free Sample
Europe Remicade Biosimilars Market Analysis and Trends
In Europe, the dominance in the Remicade Biosimilars market emanates from comprehensive supportive policies favoring biosimilar substitution and pricing controls. The European Medicines Agency (EMA) has enabled accelerated biosimilar approvals, which, alongside widespread physician and patient acceptance, have resulted in biosimilars capturing more than 60% of infliximab prescriptions in 2025. Key companies such as Samsung Bioepis and Sandoz have established manufacturing and distribution centers here, boosting market revenue and competitive dynamics.
Asia Pacific Remicade Biosimilars Market Analysis and Trends
The Asia Pacific exhibits the fastest growth in the Remicade Biosimilars market. The region's CAGR surpasses 16%, driven by government initiatives to improve accessibility in countries such as India and China, coupled with local pharmaceutical players like Biocon and Dr. Reddy’s Laboratories expanding their biosimilar portfolios. Increasing prevalence of autoimmune diseases combined with cost sensitivity among patients leads to accelerated biosimilar uptake—fueled by enhanced regulatory frameworks that parallel global standards.
Remicade Biosimilars Market Outlook for Key Countries
USA Remicade Biosimilars Market Analysis and Trends
The USA's Remicade Biosimilars market is set to witness robust expansion, powered by progressive FDA policies on biosimilar interchangeability and patent expirations of reference products. Adoption rates increased by approximately 25% in 2024, catalyzed by substantial cost savings associated with biosimilar prescriptions. Leading market players such as Pfizer and Amgen have intensified their biosimilar promotion initiatives, penetrating hospital systems and specialty clinics. The US healthcare system’s reimbursement strategies are evolving to incentivize biosimilar use, further propelling market revenue growth.
India Remicade Biosimilars Market Analysis and Trends
India's Remicade Biosimilars market is rapidly growing, with local manufacturers like Biocon and Dr. Reddy’s at the forefront, contributing to nearly 35% market share domestically. Government programs aimed at improving biosimilar access in public healthcare and evolving patent landscapes have fostered an environment conducive to growth. The country’s expanding patient base affected by autoimmune disorders and increasing awareness of biosimilars have accelerated demand, making India a significant regional hub and export base for biosimilars globally.
Analyst Opinion
Pricing dynamics stand as a critical supply-side indicator shaping the Remicade Biosimilars market growth. Post patent expiry of originator Remicade, biosimilars have entered markets with discounts ranging between 20% to 35%, substantially affecting market share. For instance, in Europe, biosimilars accounted for over 60% of infliximab prescriptions in 2024, underscoring pricing leverage as a market expansion catalyst.
Demand-side indicators spotlight growing off-label and new therapeutic use cases expanding Remicade Biosimilars uptake across autoimmune disorders beyond rheumatoid arthritis and Crohn’s disease. In 2025, infliximab biosimilar utilization surged by 18% in dermatology, driven by clinical guideline endorsements from institutions such as the American Academy of Dermatology.
Production capacity enhancements, including scale-up by prominent manufacturers, have reduced lead times and distribution bottlenecks. Notably, Asian-based producers increased their manufacturing output by nearly 25% in 2024, mirroring growing export volumes to key European and North American markets—further intensifying competitive market dynamics.
Export trends reveal expanding intraregional trade flows, especially from biosimilar hubs like India to regulated markets. Export volumes in 2024 rose 30% year-over-year due to streamlined regulatory harmonization and favorable trade policies, which significantly impact market revenue and support business growth in emerging geographies.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2025: | USD 3.8 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 13.5% | 2032 Value Projection: | USD 9.1 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Pfizer Inc., Samsung Bioepis, Celltrion, Inc., Sandoz (a Novartis division), Biocon Ltd., Amgen Inc., Mylan N.V., Dr. Reddy’s Laboratories, Zydus Cadila, Intas Pharmaceuticals, Lupin Ltd., Teva Pharmaceuticals. | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Remicade Biosimilars Market Growth Factors
The growing prevalence of autoimmune disorders and inflammatory conditions has significantly amplified the Remicade Biosimilars market demand. Epidemiological data from 2024 indicated a global rheumatoid arthritis prevalence of 3.8%, directly fueling biosimilar adoption.
Healthcare cost containment strategies across developed and emerging economies are accelerating biosimilar uptake, with governments incentivizing biosimilar prescription. For example, the NHS England reported over 40% biosimilar use in infliximab treatments by mid-2025, driven by cost-saving initiatives.
Regulatory reforms facilitating faster biosimilar approvals, such as the US FDA’s streamlined interchangeability guidelines, have shortened time-to-market and reduced entry barriers, benefiting market revenue.
Innovation in drug delivery technologies, including biosimilar formulations with improved stability and subcutaneous administration, is providing a competitive edge and expanding market scope in specialty clinics and outpatient settings.
Remicade Biosimilars Market Development
In April 2025, IXIFI (infliximab for injection), a biosimilar to Remicade developed by Pfizer, became available in Canada. Initially approved by Health Canada in December 2021 for all eligible indications of the reference product, the April 1, 2025, commercial availability marks a key step in expanding access to this infliximab biosimilar in the Canadian market.
In December 2019, Avsola (infliximab-axxq) from Amgen received U.S. Food and Drug Administration approval as a biosimilar to Remicade®, covering all the same indications, including rheumatoid arthritis, Crohn’s disease, ulcerative colitis, psoriatic arthritis, and ankylosing spondylitis.
Key Players
Leading companies of the market include:
Pfizer Inc.
Samsung Bioepis
Celltrion, Inc.
Sandoz (a Novartis division)
Biocon Ltd.
Amgen Inc.
Mylan N.V.
Dr. Reddy’s Laboratories
Zydus Cadila
Intas Pharmaceuticals
Lupin Ltd.
Teva Pharmaceuticals
Competitive strategies include Pfizer Inc.’s aggressive biosimilar pipeline diversification coupled with strategic partnerships, leading to a 15% increase in its infliximab biosimilar market penetration in North America during 2024. Samsung Bioepis enhanced its market footprint through technology transfer agreements, boosting production efficiency and generating a 20% revenue uplift from biosimilars in Asian markets. Biocon Ltd. leveraged joint ventures with glo
Remicade Biosimilars Market Future Outlook
In the future, the Remicade Biosimilars Market is expected to achieve broader global adoption as regulatory clarity improves and interchangeability standards become more uniform. Declining manufacturing costs, coupled with expanded approval of subcutaneous and combination formulations, will enhance patient convenience and compliance. Market competition will intensify as multiple biosimilars enter the pipeline, driving price erosion but expanding patient access. Emerging markets, especially in Asia and Latin America, are poised to become major growth centers due to healthcare cost containment initiatives and government biosimilar promotion policies.
Remicade Biosimilars Market Historical Analysis
The Remicade Biosimilars Market emerged as a cost-effective alternative to the branded biologic infliximab, which was widely used for autoimmune conditions such as rheumatoid arthritis, Crohn’s disease, and ulcerative colitis. The first biosimilars launched in Europe around 2015, quickly gaining traction due to supportive reimbursement frameworks and physician confidence. In contrast, U.S. adoption lagged initially because of patent litigation, contracting complexities, and interchangeability concerns. Over the past decade, biosimilar manufacturers have focused on demonstrating therapeutic equivalence, improving pharmacovigilance, and establishing competitive pricing models that gradually increased market penetration.
Sources
Primary Research Interviews:
Rheumatologists
Gastroenterologists
Hospital Pharmacists
Biologics Market Analysts
Health Policy Experts
Databases:
FDA Biologics Database
EMA Biosimilars Reports
Magazines:
Biosimilar Development
PharmaTimes
Pharmaceutical Technology
BioCentury
Journals:
The Lancet Rheumatology
Arthritis & Rheumatology
Clinical Pharmacology & Therapeutics
Biologicals
Newspapers:
Financial Times (Pharma)
The Wall Street Journal (Health)
The Guardian (Science)
The Hindu (Health)
Associations:
International Federation of Pharmaceutical Manufacturers & Associations (IFPMA)
European Medicines Agency (EMA)
American College of Rheumatology (ACR)
Biosimilar Council (USA)
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients