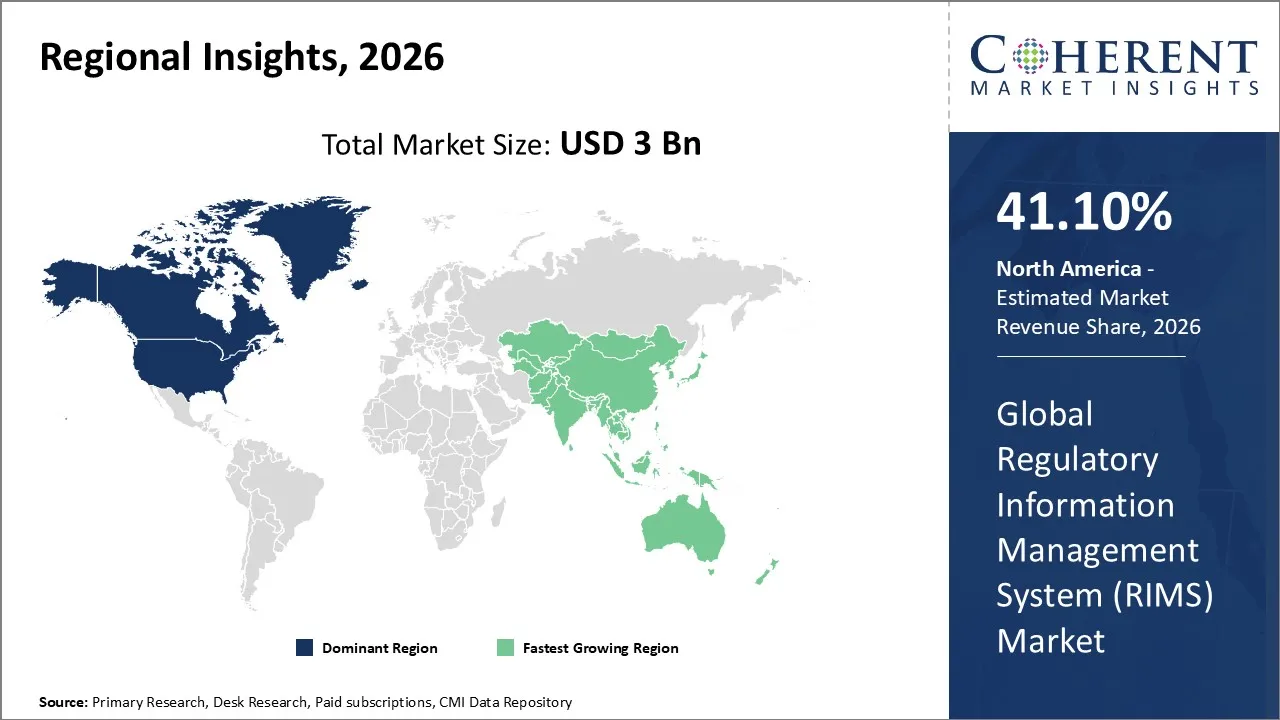
The Regulatory Information Management System (RIMS) Market is estimated to be valued at USD 3 Bn in 2026 and is expected to reach USD 6.21 Bn by 2033, exhibiting a compound annual growth rate (CAGR) of 12.7% from 2026 to 2033.
The RIMS business is getting bigger as pharmaceutical, biotech, medical device, and chemical companies have to deal with more complicated rules. Companies use RIMS to bring all their data together, make submissions easier, and improve teamwork around the world. By leveraging cloud platforms, automation, and AI, companies improve document management, track activities in real time, strengthen audit readiness, and accelerate digital transformation, making RIMS essential for regulatory compliance and product lifecycle management.
|
Current Events |
Description and its impact |
|
Geopolitical and Regulatory Landscape Shifts |
|
|
Economic and Industry-Specific Pressures |
|
|
Societal and Compliance-Driven Movements |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Artificial intelligence plays a transformative role in Regulatory Information Management (RIM) systems by automating data-intensive regulatory processes and improving accuracy and efficiency. AI enables intelligent document classification, regulatory impact analysis, and real-time tracking of global regulatory changes. By identifying patterns, inconsistencies, and risks across submissions, registrations, and commitments, AI reduces manual effort and compliance errors.
In September 2025, Rimsys, a leading Regulatory Information Management (RIM) platform for MedTech, announced the launch of Rimsys AI, a suite of embedded AI agents designed to streamline regulatory workflows and improve efficiency for global regulatory teams.
Software expected to hold largest market share of 53.6% in 2026. The RIMS software market is growing as businesses use centralized, automated solutions to make regulatory procedures easier. RIMS helps businesses keep track of submissions, manage papers, and make sure that all areas are following the rules. Cloud deployment, real-time access, and scalability make it easier for teams all over the world to work together and get things done. Workflow automation, analytics, and system integration make things more accurate and productive. For instance, in July 2024, ArisGlobal launched its new LifeSphere Regulatory platform, a unified solution that streamlines regulatory workflows while improving visibility and data quality.
Registration holds the largest market share of 43.6% in 2026. The registration part of the RIMS industry is growing because businesses use RIMS systems to handle approvals and regulatory submissions more quickly, follow new global rules, and make fewer mistakes. Digitalization, consolidated data, and better teamwork make it easier to keep track of submissions, speed up approvals, and promote transparency and adoption. For instance, PAREXEL International Corporation introduced LIQUENT InSight® 6.0, the latest version of its Regulatory Information Management platform, supporting the full regulatory product lifecycle from planning to product retirement.
Cloud-based acquired the prominent market share of 56.6% in 2026. Companies are pushing for the use of cloud-based RIMS because they want more flexibility, scalability, and cost-effectiveness. They employ cloud systems to get real-time access to regulatory data, make filings easier, and work together from different places. Easy installation, automatic upgrades, fewer IT demands, and built-in security all make things run more smoothly, keep sensitive data safe, and let businesses respond quickly to changing compliance needs. For instance, Rimsys, a leading medtech Regulatory Information Management provider, launched Rimsys 5 at the MedTech Forum in Barcelona, helping companies boost compliance and accelerate product launches.
SMEs capture the largest market share of 37.9% in 2026. Small and medium-sized businesses (SMEs) increasingly use RIMS to manage complex regulations efficiently. They centralize paperwork, track submissions, and maintain accurate records, reducing errors and workload. Cloud-based, scalable solutions enable compliance without heavy infrastructure. By automating tasks, improving collaboration, and providing real-time process visibility, RIMS helps SMEs accelerate approvals, stay compliant, and compete effectively with larger companies.
Pharmaceutical Industry holds the largest market share of 43.6% in 2026. Pharmaceutical companies increasingly use RIMS to manage complex regulations across drug development, approval, and post-market stages. They centralize submission data, track approvals, and ensure accuracy while complying with global rules. By leveraging automation, analytics, and cloud solutions, firms streamline workflows, enhance collaboration, reduce errors, accelerate submissions, improve lifecycle management, and simplify audits. For instance, in July 2025, Indegene launched NEXT Medical Writing Automation to speed up the creation of high-quality documents for clinical development, regulatory submissions, and more.
To learn more about this report, Download Free Sample
North America dominates the overall market with an estimated share of 41.1% in 2026. In North America, companies drive the RIMS market by adopting cloud, AI, and automation to streamline regulatory workflows and enhance data management. By integrating RIMS with enterprise systems, they improve submission tracking and team collaboration. Continuous innovation in intelligent tools and the demand for secure, centralized regulatory data actively shape regional market growth. For instance, Veeva Systems announced that Epredia is adopting Veeva Vault Quality and Veeva MedTech to unify quality and regulatory information, improving transparency and compliance.
Companies in the Asia Pacific region are driving the growth of the RIMS market by using digital platforms to keep up with regulations as the pharmaceutical and biotech industries grow and regulatory scrutiny increases in China, India, and Japan. More clinical trials and multinational operations mean more need for consolidated data and automated workflows. This leads to more money being spent on advanced RIMS solutions that can handle complicated regulatory requirements quickly and easily.
Companies in the U.S. run the RIMS industry by keeping up with complicated compliance and more regulatory filings in healthcare and life sciences. They use RIMS to keep all of their documents in one place, handle electronic submissions, and make sure that their procedures are in line with FDA guidelines. Companies improve submission tracking, operational efficiency, and overall regulatory process management by using cloud computing, automation, and AI.
Companies in China are the ones who drive the RIMS industry by using digital tools to keep up with new rules and higher compliance needs in the pharmaceutical and medical device industries. They utilize RIMS to make submissions easier, keep all their documents in one place, and keep track of changes to rules. As clinical trials and multinational operations grow, the need for automated workflows grows as well. This makes companies need scalable, integrated technologies to make sure they are following the rules and working efficiently.
RIMS is using more and more cloud-native solutions, which lets regulatory teams work more freely and grow more easily. Companies are getting rid of old on-premise systems in favor of remote access, easier IT maintenance, and faster upgrades from vendors. Cloud deployments focus on high security, multi-tenant designs, and flexible subscription models. This makes it possible for enterprises of all sizes to employ advanced regulatory technology without having to pay a lot of money up front for infrastructure.
RIMS increasingly integrates with quality management, document control, and product lifecycle systems to eliminate duplicate data and manual checks. End-to-end traceability from filings to compliance actions enables regulatory, quality, safety, and production teams to collaborate effectively. This integrated approach ensures regulatory requirements and organizational processes stay aligned throughout the entire product lifecycle, providing a comprehensive view of compliance at every stage.
Sector-specific regulations in cosmetics, chemicals, biotech, and medical devices are increasing, and generic RIMS platforms often fail to address unique compliance challenges. Vendors can create tailored modules to handle product classification, submissions, audits, and labeling for specific industries. Customized RIMS features help businesses deliver more value, accelerate adoption, and strengthen customer loyalty in targeted sectors.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 3 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 12.7% | 2033 Value Projection: | USD 6.21 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Acuta, LLC, AMPLEXOR, ArisGlobal LLC, arivis AG, DDi, Inc., Ennov SA, Extedo Gmbh, GLEMSER TECHNOLOGIES CORPORATION, Samarind Ltd., Sparta Systems Inc., Veeva Systems, and Virtify, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Ankur Rai is a Research Consultant with over 5 years of experience in handling consulting and syndicated reports across diverse sectors. He manages consulting and market research projects centered on go-to-market strategy, opportunity analysis, competitive landscape, and market size estimation and forecasting. He also advises clients on identifying and targeting absolute opportunities to penetrate untapped markets.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients