Global regulatory affairs outsourcing market is estimated to be valued at USD 9.33 Bn 2025 and is expected to reach USD 19.26 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 10.9% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
Regulatory compliance is becoming more and more important with increasing regulations and the market is witnessing significant growth due to rising outsourcing of regulatory functions by medical device and pharmaceutical companies.
Growing clinical trial activities and new product launches can boost demand for outsourcing regulatory services. Moreover, increasing stringent regulatory standards prompt life sciences companies to rely more on specialist regulatory consultancies for ensuring compliance.
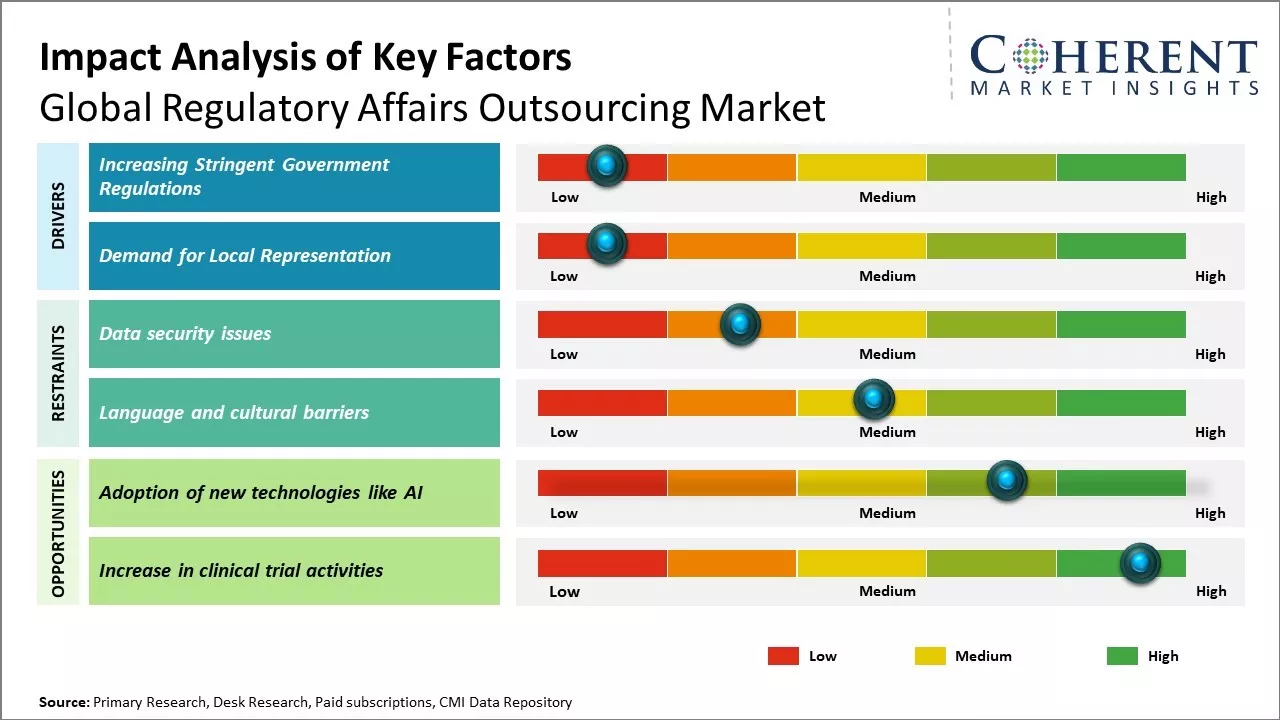
Increasing Stringent Government Regulations
With the rapid development of new technologies, drugs and medical devices, regulatory frameworks and guidelines have become more complex and stringent. Pharmaceutical and biotech companies are finding it challenging to continuously keep track of the dynamic regulatory landscape and ensure compliance at all stages of product development and marketing. The approval processes have become lengthier with increased data requirements. Any oversight can delay the launch of new products and hinder business operations. Outsourcing regulatory work allows companies to leverage the dedicated expertise of specialized providers that continually monitor changes in regulations. This helps them navigate the complex compliance requirements more efficiently and mitigate risks of non-compliance. Subject matter experts at outsourcing firms keep abreast of the evolving guidelines through constant research and stakeholder interactions. These can proactively guide companies and ensure all submissions meet current standards, thus, accelerating approval timelines. Outsourcing non-core regulatory functions frees up internal resources that can be better utilized to focus on strategic business priorities.
Get actionable strategies to beat competition: Download Free Sample
Demand for Local Representation
With rise of new markets globally, companies increasingly aim to expand into international geographies. However, regulatory compliance and product approval processes differ widely across countries and regions due to variances in local healthcare systems, policies, and cultural contexts. Meeting individual country-level requirements requires in-depth knowledge of local regulations as well as infrastructure like facilities and personnel on-ground. This poses organizational challenges for multinational corporations managing global operations. Regulatory outsourcing provides them access to dedicated country experts who understand local regulatory nuances. Outsourcing partners establish local offices and hire native representatives who can directly liaise with the relevant authorities on their behalf. These ensure that all documentation for approval is exactly as per the local filing standards. This eases market entry by effectively navigating domestic complexities and expediting approvals. Companies can leverage outsourcers' existing set-up across various regions to augment their international presence without the need for separate investments. Local outsources partners play an instrumental role in helping life sciences firms expand into new high potential markets worldwide.
Key Takeaways from Analyst:
Global regulatory affairs outsourcing market is expected to witness growth. North America currently dominates the market due to stringent regulations around drug development and medical devices. However, Asia Pacific is expected to be fastest growing region due to lower costs and growing in-house capabilities of regulatory service providers (RSPs) in the region.
The market growth is driven by biopharma industry's need to focus on core competencies and cost savings achieved through outsourcing regulatory work. Navigating the complex regulatory landscape requires specialized expertise that most companies lack internally, thus, boosting need to engage external experts. Compliance requirements are also becoming more complex due to global nature of clinical trials and product registrations.
Data privacy and information security concerns can hamper the market growth. Regulatory submissions involve exchange of critical product information and compliance data, which needs robust protection. However, outsourcing reduces control over intellectual property and compliance strategies. Changing regulations and disruptions like the COVID-19 pandemic increase uncertainty.
Companies are increasingly demanding value-added and strategic services like regulatory intelligence, strategy advisory and end-to-end submissions management.
Market Challenges: Data security issues
Data security issues can hamper the global regulatory affairs outsourcing market growth. Regulatory affairs outsourcing involves sharing of sensitive information such as intellectual property, clinical trial data, product approval strategies and compliance documents with outsourcing companies. Any breach of data security can compromise such sensitive information and harm the business and reputation of pharmaceutical/medical device companies. With increasing number of cyber threats and data breaches, companies have become more cautious about data sharing. Several incidences have been reported, where regulatory documents were leaked or stolen due to poor data security of outsourcing companies. For instance, according to a report published by U.S. Food and Drug Administration in 2021, few biotech startup companies faced regulatory delays and monetary losses after their product approval documents were compromised from outsourcing company servers. This eroded customer confidence in outsourcing. Furthermore, strict data localization and residency laws in many countries discourage cross-border data sharing. Companies located in jurisdictions like China are especially a high risk from data security perspective. The growing data privacy regulations worldwide have also increased compliance requirements for outsourcing companies to strengthen their data security infrastructure and practices.
Market Opportunities: Adoption of new technologies like AI
Rising use of advanced technologies like artificial intelligence (AI) and machine learning (ML) can offer regulatory affairs outsourcing market growth opportunities. These tools have the potential to revolutionize how compliance work is done - making workflows more efficient, automated and data-driven. AI can analyze huge volumes of documents, data and files much faster than humans. It can help accelerate regulatory document drafting, submissions management, labelling changes and stay updated with global regulatory changes instantly. This could free up regulatory professionals to focus more on high-level strategic work rather than routine tasks. Implementing AI technologies requires significant resources and expertise. However, regulatory outsourcing firms are well positioned to lead this transition due to their scale of operations, technical know-how, and access to global talent. By investing in new technology hubs and skill development, companies can build comprehensive AI-driven regulatory solutions and platforms. This will allow them to offer more specialized services and take on larger work portfolios from biotech and pharmaceutical clients. As regulation becomes more complex across sectors like healthcare, consumer goods, IT and energy due to geopolitical factors, there will be huge demand for digital transformation.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
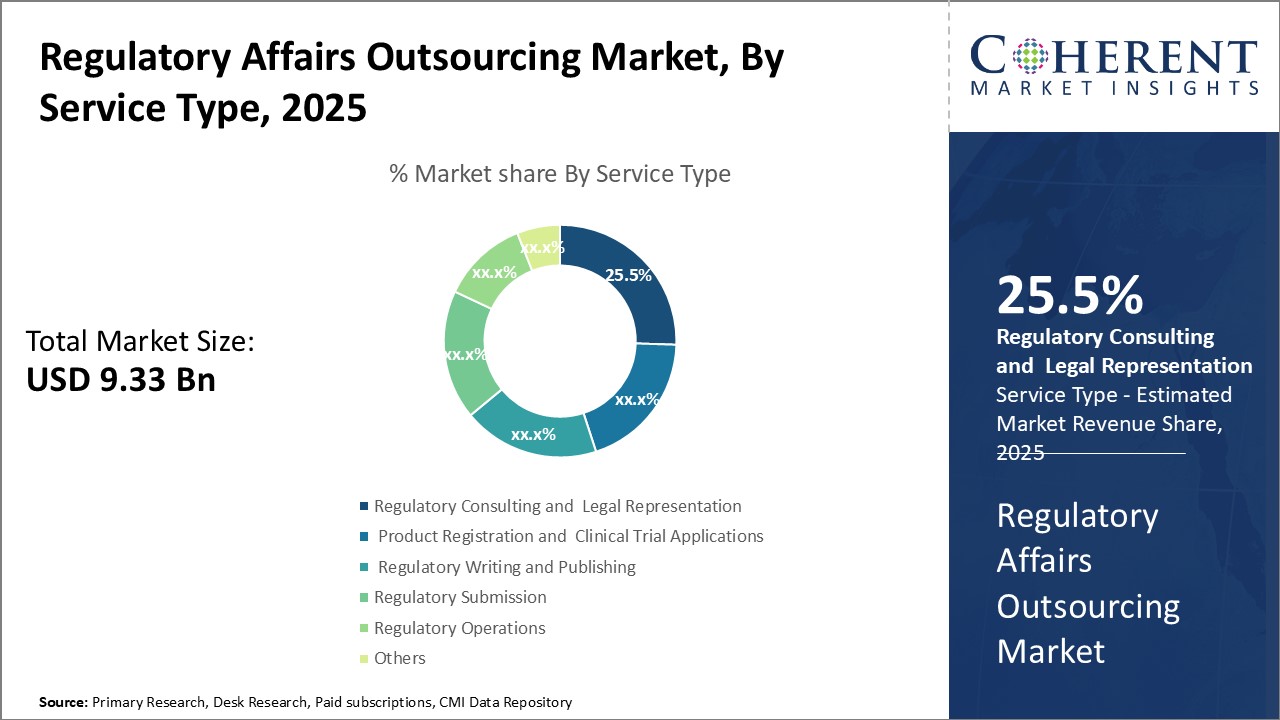
By Service Type - Expertise and Experience Boosts Demand for Regulatory Consulting
In terms of service type, regulatory consulting and legal representation segment is estimated to contribute the highest market share of 25.5% in 2025, owing to the need for specialized expertise. Providing regulatory consulting and legal advice involves intricate knowledge of the ever-changing rules and regulations that govern clinical research and product approval processes. It also requires experience navigating the complex submission requirements and guidelines of health authorities. For many companies, especially small to mid-sized biotechs and medtech firms, it is not feasible to build and maintain an in-house team with the breadth of regulatory know-how needed across multiple jurisdictions. Outsourcing to specialized regulatory consultancies allows companies to leverage the expertise of regulatory professionals who do this work day-in and day-out. Consultants stay up-to-date on the latest policy changes and recommendations of agencies like the FDA and EMA. These can efficiently guide sponsors through regulatory procedures and help interpret agency feedback. Using external advisors also provides an unbiased perspective separate from internal pressures or politics. This is particularly important for legal evaluations and negotiations with regulators. The consultative services help minimize compliance risks and keep development programs on the most expeditious approval path. For large pharmaceutical firms, regulatory consulting provides surge capacity and additional geographic coverage without requiring new hires. Mid-sized and start-up biotechs rely on consultants for their very ability to navigate global approval processes and bring important new therapies to patients. As the regulatory landscape grows ever more complex, there will be huge demand for advisory services that distill expert regulatory knowledge into actionable guidance.
By End User, Pharmaceutical Companies contributes the highest share of the market owing to their extensive regulatory requirements
By end user, pharmaceutical companies segment is estimated to contribute the highest market share of 50.5% in 2025, face some of the most stringent regulatory requirements compared to other industries in the healthcare sector. Given the critical nature of drugs and their potential safety impacts, pharmaceutical products need to undergo rigorous clinical trials and obtain numerous regulatory approvals before these can be commercialized. Compliance with regulations is a complex process that requires dedicated expertise across multiple domains such as clinical research, product registration, safety monitoring, and compliance. The regulatory landscape also continuously evolves with new guidelines being issued. Managing regulatory requirements internally requires significant investments in building and continuously updating internal regulatory capabilities. For pharmaceutical companies, non-compliance with regulations can lead to delays in product approvals, rejection of applications, financial penalties, and even withdrawal of approved products in some cases. Given the high stakes, most pharmaceutical companies prefer to outsource their regulatory requirements to specialized contract research organizations that have deep regulatory expertise.
By Stage - Clinical contributes the highest share of the market
By stage, clinical segment is estimated to contribute the highest market share of 51% in 2025. Clinical testing is a critical stage in the drug and device development process that involves several regulatory requirements. Once a product enters clinical trials, the developer needs to comply with extensive regulations across areas such as trial protocols, safety monitoring, informed consent processes, data management and reporting. Dependable execution of clinical trials as per global regulatory standards is important to demonstrate a product’s safety and efficacy profile. However, designing and managing clinical programs as per constantly evolving guidelines requires substantial expertise that most industry players lack internally. Even larger companies find it difficult to have capabilities for all geographic and therapeutic regions. Non-compliance during clinical trials can delay further development and even risk trial sanctions. Given the costs and stakes involved, developers increasingly prefer to outsource clinical trial activities and associated regulatory functions. Outsourcing to specialized clinical research organizations with global regulatory capabilities allows them to stay focused on product development while ensuring compliance. It also provides access to a global network of investigators and sites. Many regulations require local representation and understanding of cultural sensitivities for trials conducted in international regions. Regulatory service providers help bridge these barriers by providing globally distributed resources with local expertise.
Need a Different Region or Segment? Download Free Sample
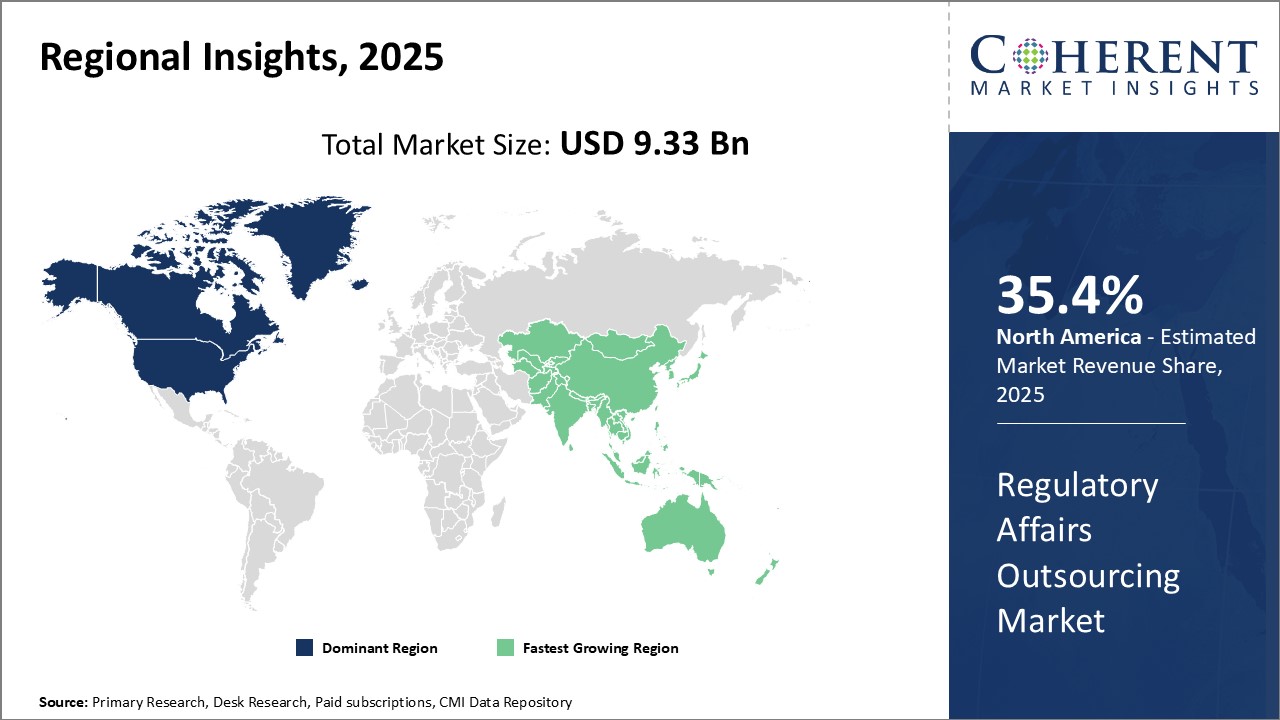
North America dominates the regulatory affairs outsourcing market with an estimated market share of 35.4% in 2025. With the presence of well-established pharmaceutical companies and robust regulations, North America accounted for over 35.5% of the global market share in 2021. The region is home to major hubs like U.S. and Canada that boosts demand for outsourcing regulatory functions. Stringent regulations such as FDASIA have necessitated companies to rely on specialized regulatory service providers for ensuring compliance. Presence of leading contract research organizations (CROs) offering end-to-end regulatory solutions drives the market growth. Furthermore, high concentration of clinical trials in this region increases the need for regulatory expertise.
Asia Pacific region is poised to be the fastest growing market during the forecast period. Countries like China, India and Japan are emerging as global sourcing destinations for regulatory affairs due to increasing investments in healthcare infrastructure and pharmaceutical manufacturing capabilities. Supportive government policies promoting the life sciences industry coupled with availability of low-cost regulatory skilled resources are encouraging companies to establish regional hubs in Asia. Rising preference for Asia Pacific CROs owing to their comprehensive capabilities and localized expertise can boost outsourcing activity in this region. In addition, Asia Pacific companies are actively enhancing their global regulatory networks to cater growing international marketing authorizations for their products. While pricing of services is relatively lower compared to mature markets, the region offers high future growth potential backed by improving quality standards.
Regulatory Affairs Outsourcing Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 9.33 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.9% | 2032 Value Projection: | USD 19.26 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
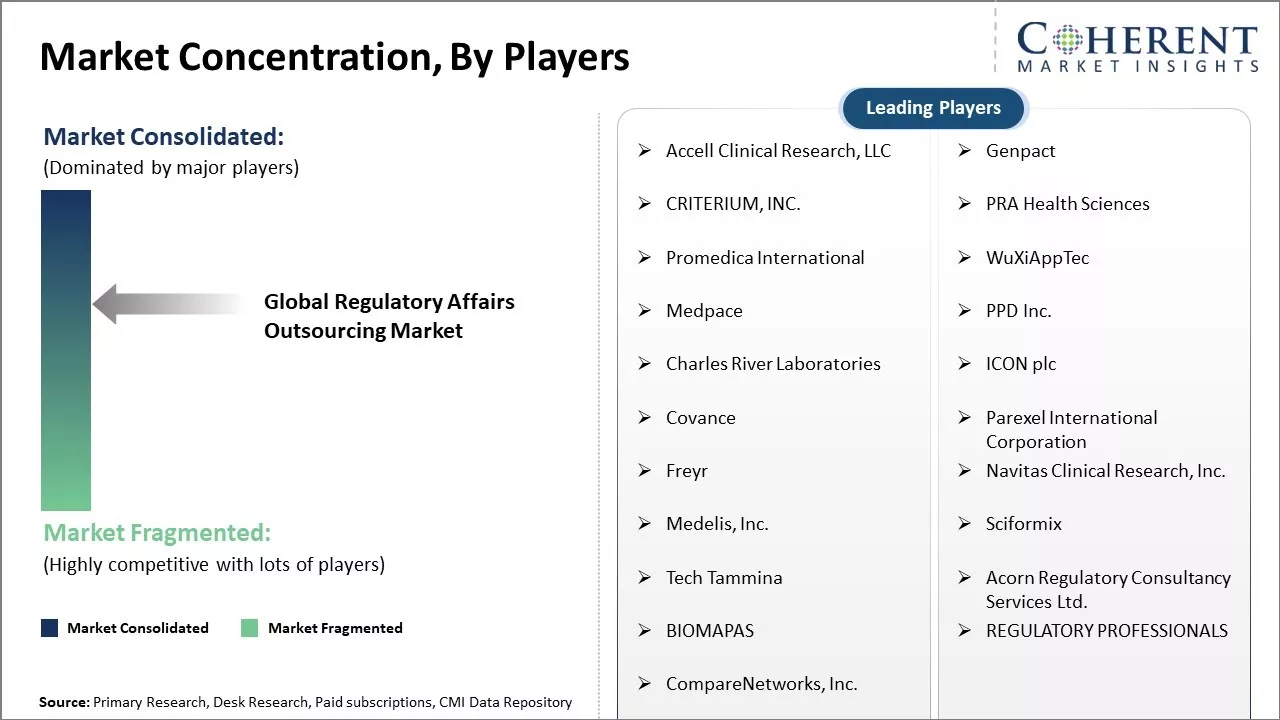
Accell Clinical Research, LLC, Genpact, CRITERIUM, INC., PRA Health Sciences, Promedica International, WuXiAppTec, Medpace, PPD Inc., Charles River Laboratories, ICON plc, Covance, Parexel International Corporation, Freyr, Navitas Clinical Research, Inc., Medelis, Inc., Sciformix, Tech Tammina, Acorn Regulatory Consultancy Services Ltd., BIOMAPAS, REGULATORY PROFESSIONALS, CompareNetworks, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: Regulatory Affairs Outsourcing Market provides regulatory services to help life sciences and medical device companies comply with the various regulatory requirements of health authorities globally. The services include assisting with the development and submission of documentation for approval of drugs, biologics and medical devices. Companies outsource their regulatory functions to specialized service providers to manage the complex regulatory environment more cost-effectively and focus internal resources on core business activities like R&D and commercialization. Outsourcing regulatory services allows companies to stay compliant with regulations and bring products to market faster.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients