Regenerative artificial skin market is estimated to be valued at USD 3,084.9 Mn in 2026 and is expected to reach USD 5,980.0 Mn by 2033, exhibiting a compound annual growth rate (CAGR) of 9.9% from 2026 to 2033.
Rising prevalence of chronic wounds, burn injuries, and surgical trauma globally is driving the demand for regenerative artificial skin solutions. The demand for advanced products for healing wounds, such as bioengineered and cellular skin substitutes, is increasing in hospitals, burns, and specialized clinics because of their ability to promote quick recovery and ensure prevention of any further complications.
Company leaders are working on introducing innovative products for skin construction with greater compatibility and functionality for handling both acute and chronic conditions associated with wounds. Rising investment in the field of regenerative medicine and increased healthcare infrastructure and awareness about advanced technology in the field of wounds are boosting the market.
|
Current Events |
Description and its impact |
|
Advanced Bioprinting and Tissue Engineering Breakthroughs |
|
|
U.S.-China Trade Relations and Biotechnology Competition |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
By material type, the Biological Materials segment is projected to hold the largest market share of 34% in 2026. This can be attributed to the excellent biocompatibility attribute of Biological Materials. Biological scaffolds such as collagen and fibrin have very similar characteristics to natural human skin; hence, they are easily integrated into the body tissues and are resistant to rejection. Hospitals and specialized centers use these materials to treat both acute burn wounds as well as chronic wounds.
For instance, in October 2025, researchers from the Mayo Clinic announced the development of a fully humanized 3D bioprinted human skin model that uses CollPlant Biotechnologies’ plant‑derived recombinant human collagen (rhCollagen) bioink.
By product type, the Bioengineered Skin segment is projected to have the largest market share of 31% in 2026. This is due to the enhanced wound healing and functional performance of Bioengineered Skin compared to conventional graft substitutes. Bioengineered products usually incorporate cellular elements along with synthetic or biological scaffolds.
For instance, in April 2025, AVITA Medical introduced Cohealyx, a bioengineered collagen‑based skin matrix designed to reduce preparation time and improve graft integration in complex wound and burn treatments.
Based on application, the Burn Treatment & Management segment is estimated to command a share of 47% in 2026 owing to the immense demand for superior burn management solutions. Burn centers and hospitals demand superior artificial skin products capable of handling acute cases of burns effectively.
For instance, In May 2025, CUTISS AG announced progress in its Phase 3 clinical trial of denovoSkin™, a personalized bio‑engineered skin graft designed for severe burn patients, with initial grafts completed at clinical sites in multiple EU countries.
Based on the end-user, the Hospitals segment is anticipated to lead the market in 2026, holding a share of 58%, as it is primarily used as the channel through which advanced regenerative artificial skins are adopted because of its specialized infrastructure as well as its qualified medical staff.
For instance, In September 2025, AVITA Medical announced that real‑world clinical data demonstrated its RECELL® System significantly reduced hospital length of stay by an average of 36% for burn patients compared with traditional skin grafting.
To learn more about this report, Download Free Sample
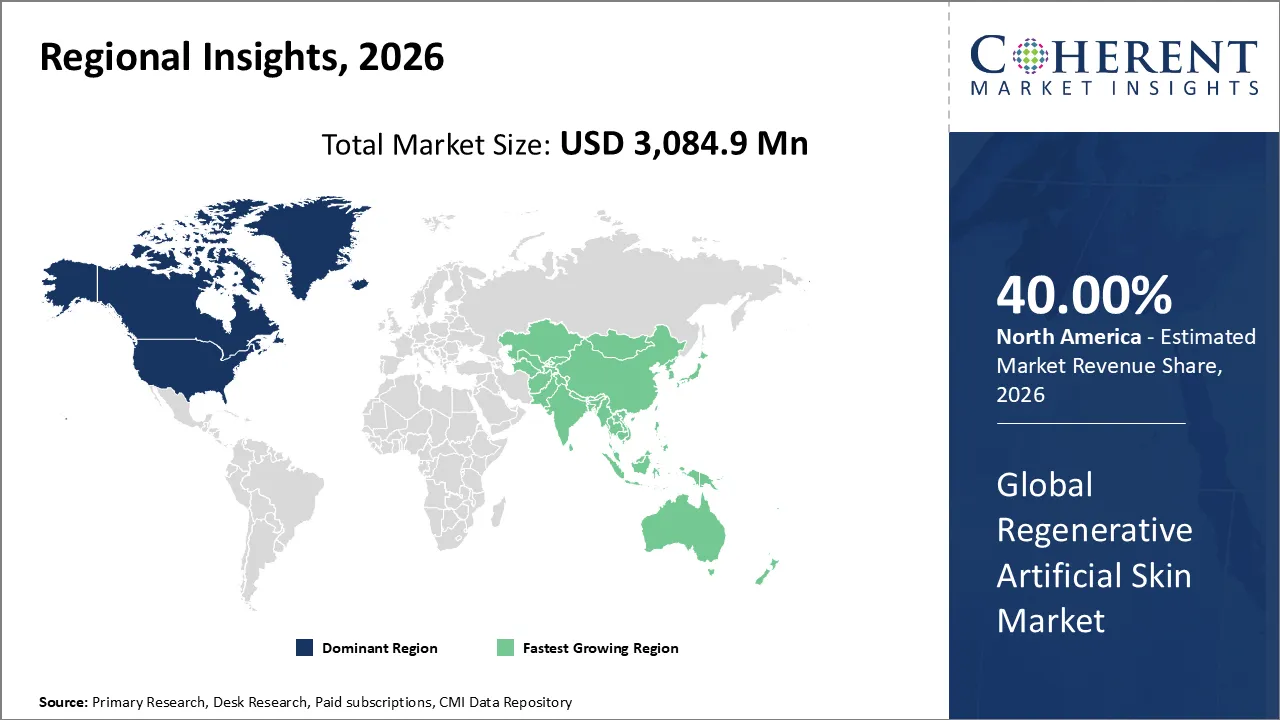
North America is projected to record the highest market share of 40% in the regenerative skin market by 2026. This is due to factors such as an extensive health care infrastructure, a large adoption rate of advanced wound care technology, and presence of major market influencers who are actively engaging in R&D activities for regenerative medicine.
For instance, in June 2025, AVITA Medical announced that its RECELL® System and RECELL® GO were recognized as the “Best New Technology Solution, Surgical” at the 2025 MedTech Breakthrough Awards, highlighting leadership in regenerative skin care technologies used in hospitals and burn treatment centers across North America.
The Asia Pacific region is expected to be the fastest-growing market due to increasing healthcare investments, growing prevalence of burns and chronic wounds, and a growing awareness about advanced wound management products entering the market. Rising investment in the infrastructure related to hospitals in countries such as China, India, and Japan, and an environment that is conducive to regenerative medicine, is driving the demand for bioengineered skin substitutes in the Asia Pacific region.
For instance, in May 2025, CUTISS AG advanced its denovoSkin™ Phase 3 clinical trial across multiple EU and Asia Pacific clinical sites, including burn centers in India and Japan, demonstrating the region’s growing focus on adopting next-generation bioengineered skin solutions.
The U.S. regenerative artificial skin market is growing rapidly, driven by high adoption of advanced wound care solutions, well-established healthcare infrastructure, and supportive regulatory frameworks for regenerative medicine. Hospitals and burn units are gradually adopting bio-engineered artificial skins as an adjunct for quicker patient recovery.
For instance, In June 2025, AVITA Medical announced that its RECELL® Platform, including RECELL and RECELL GO, was honored as the “Best New Technology Solution, Surgical” at the 2025 MedTech Breakthrough Awards.
China’s regenerative artificial skin market is growing steadily, promoted by an increasingly developed healthcare infrastructure, encouraging government initiatives concerning innovative and advanced solutions for wound management, as well as an increasing number of instances related to burns or chronic wounds.
For instance, in October 2025, international dermatology company Galderma introduced four core Alastin® products in the Chinese market designed to support natural skin regeneration around aesthetic treatments, helping peri‑ and post‑procedure recovery.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 3,084.9 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 9.9% | 2033 Value Projection: | USD 5,980.0 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Integra LifeSciences Corporation, Smith & Nephew plc, Organogenesis Inc., MiMedx Group, Inc., Avita Medical, Inc., Stratatech (a Mallinckrodt Company), Vericel Corporation, B. Braun Melsungen AG, Kerecis (Coloplast subsidiary), PolarityTE, Inc., Tissue Regenix Group plc, Celularity. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Rising incidence of chronic wounds, burns, and trauma injury cases is triggering the demand for regenerative artificial skin. Hospitals, burn units, and specialty care facilities are increasingly using bio-engineered and artificial skin substitutes. Rising awareness about advanced regenerative medicines among healthcare providers and consumers alike is also resulting in increased market growth.
The market also offers significant opportunities for growth in the development of next-generation bioengineered skin, stem-cell-based constructs, and composite materials. The key strategies for companies could be the formulation of faster-healing, cost-effective, and tunable alternatives for applications ranging from burn care to chronic wound management. Further, expanding into emerging markets and partnering with hospitals and research institutes can accelerate adoption and revenue growth.
The regenerative artificial skin market is revolutionizing the treatment of burns and wound care through the integration of the latest bioengineering, cell therapies, and biomaterials. The acceptance rate in hospitals and burn units is increasing, as they realize the benefits of faster healing achieved through bioengineered and stem cell-based skin substitutes. The use of products like Apligraft, Dermagraft, or RECELL is improving the process of wound healing, while at the same time streamlining operations.
The “functional skin construct” approach to recreating the structure and sensation of the epidermis represents a transition from covering the wound temporarily to a fully regenerative approach. Technologies such as fish skin grafts are gaining recognition as a means of rapid healing and reduced infection rates, reflecting the market trend shifting from purely cosmetic substitutes to something meaningful from a clinical perspective.
Geographically, the adoption is strong in regions with supportive regulatory frameworks and established healthcare infrastructure, while emerging markets are rapidly increasing their uptake through collaborations between international regenerative companies and local hospitals. It indicates a growing awareness among healthcare providers and expanded access to advanced treatments for more patients.
The market’s trajectory is driven by continuous product innovation, growing clinical confidence in regenerative therapies, and increasing integration of regenerative solutions into mainstream medical practice. The firms that work on clinically justified and cost-effective solutions are expected to drive the next wave of growth in this dynamic and revolutionary market.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients