Refractory Glaucoma Treatment Market Size and Forecast – 2026 – 2033
The Global Refractory Glaucoma Treatment Market size is estimated to be valued at USD 1.4 billion in 2026 and is expected to reach USD 2.3 billion by 2033, exhibiting a compound annual growth rate (CAGR) of 7.4% from 2026 to 2033.
Global Refractory Glaucoma Treatment Market Overview
The Refractory Glaucoma Treatment Market focuses on therapies for glaucoma cases that remain uncontrolled despite maximum medical or surgical intervention. Refractory glaucoma often requires advanced treatment approaches due to persistent elevation of intraocular pressure and high risk of vision loss. The market includes pharmaceutical therapies, glaucoma drainage devices, minimally invasive glaucoma surgeries (MIGS), laser treatments, and cyclodestructive procedures. Growth is driven by the rising global prevalence of glaucoma, aging populations, and increasing demand for effective solutions for treatment-resistant cases. Continuous technological advancements and improved surgical outcomes are further supporting market expansion worldwide.
Key Takeaways
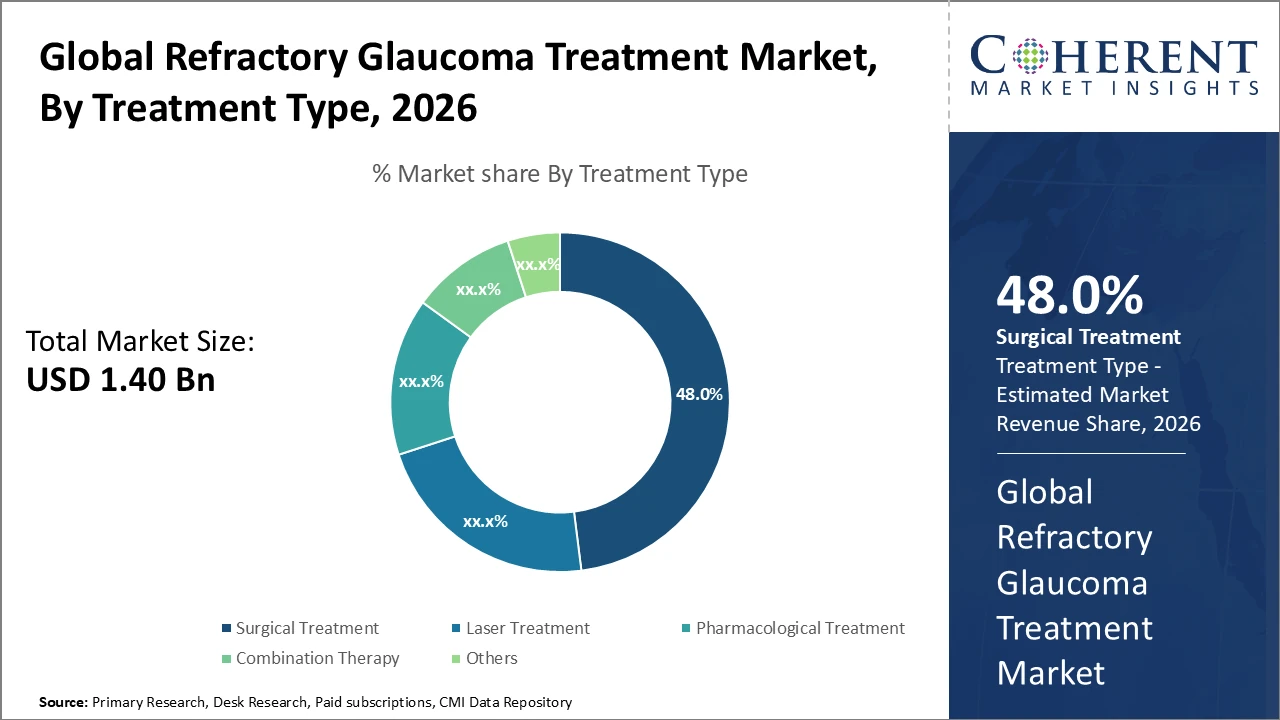
The surgical treatment segment dominates the market with a 48% share, driven by innovations in drainage implants and micro-stents that offer improved safety profiles and better clinical outcomes, making it the preferred choice among eye care practitioners.
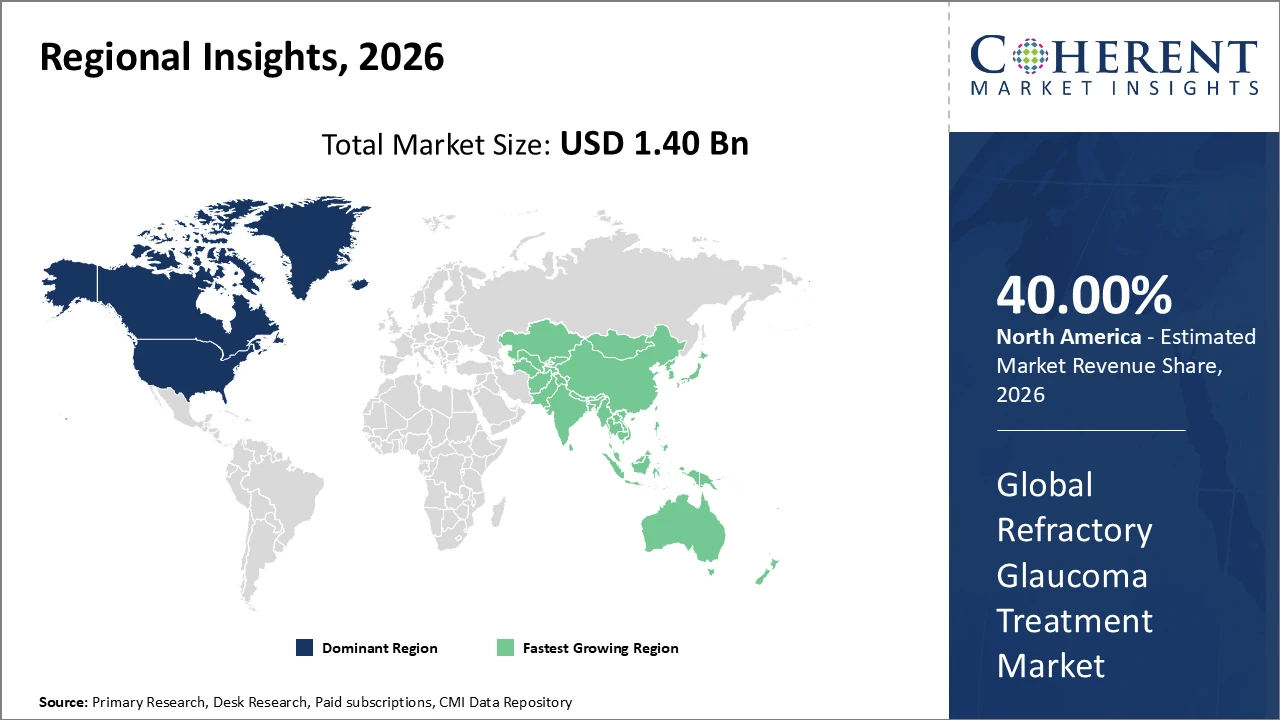
North America accounts for over 40% of the market share, supported by strong healthcare infrastructure and increased research and development investments from leading players such as Alcon and Glaukos.
Asia Pacific is the fastest-growing regional market, with a CAGR exceeding 9%, driven by a growing elderly population and expanding ophthalmology care networks in countries such as China and India.
Refractory Glaucoma Treatment Market Segmentation Analysis
To learn more about this report, Download Free Sample
Refractory Glaucoma Treatment Market Insights, By Device Type
Drainage devices dominate the market due to their proven efficacy in significantly reducing intraocular pressure in refractory glaucoma cases and their wide clinical acceptance. Trabecular micro-bypass stents are the fastest-growing subsegment, driven by continuous technological advancements and simplified implantation techniques that enhance patient safety and surgical efficiency. Cyclodestructive devices are primarily used in advanced or end-stage refractory glaucoma where other interventions have failed. Filtering devices continue to address the needs of specific patient groups, particularly those with chronic glaucoma. Additionally, other emerging devices under clinical development aim to introduce alternative mechanisms for effective ocular pressure control.
Refractory Glaucoma Treatment Market Insights, By Treatment Type
Surgical treatment holds the largest market share due to continuous innovations in drainage devices and glaucoma stents that improve surgical outcomes while minimizing complications. Laser treatment is the fastest-growing subsegment, driven by its non-invasive nature and improved precision in managing refractory glaucoma. Pharmacological treatment remains essential as an adjunct therapy, although its growth is limited by patient adherence challenges. Combination therapy is gaining adoption in severe and complex cases, representing an emerging niche within the market. Other treatment approaches include experimental and complementary therapies that are currently in various stages of development.
Refractory Glaucoma Treatment Market Insights, By End-User
Hospitals dominate the market share due to their comprehensive treatment capabilities, availability of advanced surgical infrastructure, and high patient throughput. Ambulatory surgical centers represent the fastest-growing subsegment, driven by lower operational costs, shorter patient turnaround times, and a growing preference for outpatient procedures. Specialty clinics serve niche patient populations by offering targeted and specialized glaucoma therapies. Research institutes play a supportive role in the market by conducting clinical studies and driving developmental innovations that help shape evolving treatment paradigms.
Refractory Glaucoma Treatment Market Trends
The refractory glaucoma treatment market is increasingly shifting toward personalized medicine, with greater integration of digital health technologies for continuous patient monitoring and management.
Clinical trials conducted in 2026 have demonstrated improved outcomes from combining pharmacological treatments with surgical interventions, indicating a growing trend toward hybrid therapy models.
AI-powered diagnostic tools are gaining momentum to support early detection and optimized treatment planning, enhancing clinical decision-making.
Pilot programs in Japan and South Korea have reported improved patient outcomes through the use of AI-driven diagnostics and monitoring solutions.
There is a rising preference for minimally invasive procedures, as both patients and clinicians prioritize enhanced safety, reduced complications, and faster recovery times.
These evolving trends are expected to reshape the market landscape by improving treatment efficacy and expanding access to advanced care on a global scale.
Refractory Glaucoma Treatment Market Insights, By Geography
To learn more about this report, Download Free Sample
North America Refractory Glaucoma Treatment Market Analysis and Trends
In North America, dominance in the Refractory Glaucoma Treatment Market is driven by a well-established healthcare ecosystem, strong research and development activity, and high levels of patient awareness. The United States accounts for the majority of regional market revenue, supported by leading companies such as Alcon and Glaukos that continue to drive technological innovation. Additionally, government initiatives promoting ocular health and expanded reimbursement policies have strengthened treatment accessibility, contributing to over 40% of the global market share and sustaining North America’s leadership position.
Asia Pacific Refractory Glaucoma Treatment Market Analysis and Trends
The Asia Pacific region is experiencing the fastest growth in the Refractory Glaucoma Treatment Market, driven by demographic shifts such as a rising elderly population and expanding healthcare access in countries like China and India. The market’s CAGR exceeds 9%, supported by favourable government policies, growing ophthalmic infrastructure, and increasing patient affordability. The expansion of specialty ophthalmology clinics, along with rising investments from market players, is further accelerating business growth and enhancing the region’s presence in the global market.
Refractory Glaucoma Treatment Market Outlook for Key Countries
USA Refractory Glaucoma Treatment Market Analysis and Trends
The U.S. refractory glaucoma treatment market is marked by rapid adoption of advanced technologies and extensive clinical trial activity. Leading companies maintain a strong presence, with Glaukos Corporation reporting a 20% increase in procedure adoption in 2025, driven by the launch of innovative devices. Government funding for glaucoma research and widespread insurance coverage further support market revenue growth. A competitive landscape fosters continuous innovation, positioning the U.S. as a key contributor to global industry trends and setting benchmarks for treatment standards and technological advancements.
Germany Refractory Glaucoma Treatment Market Analysis and Trends
The refractory glaucoma treatment market in Germany is driven by a growing prevalence of glaucoma among its aging population, with adults over 40 showing an increasing risk. The surgical devices segment, including drainage implants, leads local revenue and is projected to grow steadily, reflecting widespread adoption of advanced surgical options. Minimally invasive glaucoma surgery (MIGS) devices, particularly stents, are experiencing rapid growth, as clinicians increasingly favour modern procedures over traditional surgeries. The market benefits from Germany’s high healthcare standards, strong ophthalmology infrastructure, and active R&D, with both local and global players competing. Additionally, the integration of digital health tools and early diagnostic technologies is enhancing patient outcomes and shaping treatment trends.
Analyst Opinion
The growing aging population, particularly in developed countries such as the U.S. and Germany, is a key driver of demand. In 2024, over 65 million individuals worldwide were affected by glaucoma, with approximately 10–15% representing refractory cases, underscoring the need for advanced treatment options tailored to complex conditions.
Technological innovations, especially in minimally invasive glaucoma surgeries (MIGS) and novel laser therapies, are transforming market dynamics. In 2025, MIGS device adoption in North America increased by over 25%, reflecting a shift toward safer, less invasive treatments with shorter recovery times.
Supply-side expansion through increased production capacities by major medical device manufacturers has lowered treatment costs, enhancing accessibility. For example, manufacturing output of glaucoma drainage devices rose by 18% in 2026 compared to 2024, boosting affordability and market penetration.
In the Asia-Pacific region, diverse reimbursement policies and investments in healthcare infrastructure have driven market growth. China and India saw a combined 22% rise in treatment adoption in 2025, supported by improved insurance coverage and government initiatives addressing ocular health.
Market Scope
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 1.4 billion |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 7.4% | 2033 Value Projection: | USD 2.3 billion |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: | Glaukos Corporation, Alcon, Ivantis Inc., Horizon Therapeutics, Nova Eye Medical, MicroSurgical Technology Inc., EyeTech Care, Optonol Ltd, Eyenovia Inc., IOPtima Ltd., STAAR Surgical Company | ||
| Growth Drivers: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Refractory Glaucoma Treatment Market Growth Factors
The growing prevalence of complex glaucoma cases that are resistant to conventional therapies is a key driver of global demand for refractory glaucoma treatment solutions. In 2025, the World Health Organization reported a 12% year-over-year increase in glaucoma-related vision loss, highlighting the urgent need for advanced interventions. Technological innovations, including minimally invasive glaucoma surgeries (MIGS) and customizable drug delivery systems, are boosting adoption by improving clinical outcomes while minimizing adverse effects. Favorable reimbursement policies in North America and Europe have expanded patient access, and awareness campaigns promoting early detection have further driven market growth, particularly in developed regions where timely intervention is essential to preserve vision.
Refractory Glaucoma Treatment Market Development
In December 2025, Iridex announced a new thermal dynamics study validating MicroPulse and continuous-wave laser therapies for glaucoma. The findings strengthen clinical evidence and market potential, highlighting MicroPulse’s safer, titratable approach for refractory glaucoma patients. This advancement supports innovative treatment options, offering improved management for cases resistant to conventional therapies and medications.
In August 2025, The FDA cleared Myra Vision’s titratable glaucoma therapy for a U.S. IDE study. Designed for refractory glaucoma patients, the device allows adjustable treatment to better control intraocular pressure. This marks a significant step toward innovative, customizable care for those unresponsive to conventional therapies, advancing glaucoma management options.
Key Players
Leading Companies of the Market
Alcon
Glaukos Corporation
Ivantis, Inc.
Horizon Therapeutics
Nova Eye Medical
MicroSurgical Technology Inc.
EyeTech Care
Optonal Ltd.
Eyenovia Inc.
IOPtima Ltd.
STAAR Surgical Company
Competitive strategies among market players focus primarily on product innovation and strategic collaborations. For instance, Glaukos Corporation expanded its market share in 2025 by launching a next-generation trabecular micro-bypass stent, resulting in a 15% revenue increase in the U.S. within the first year. Likewise, Alcon strengthened its global presence by entering emerging markets through localized manufacturing partnerships, which contributed to a 20% growth in Asia-Pacific sales reported in early 2026. These strategies highlight the emphasis on innovation, market expansion, and strategic alliances to drive growth and maintain competitive advantage in the refractory glaucoma treatment market.
Refractory Glaucoma Treatment Market Future Outlook
The future outlook for the refractory glaucoma treatment market is highly positive, driven by rising prevalence of complex glaucoma cases and technological advancements. Adoption of minimally invasive glaucoma surgeries (MIGS), sustained-release drug delivery systems, and hybrid therapy models is expected to increase due to improved safety and long-term intraocular pressure control. Integration of AI-powered diagnostics and digital health tools will support early detection, personalized treatment, and better patient monitoring. Favorable reimbursement policies in developed regions and expanding healthcare infrastructure in emerging markets will enhance accessibility. Overall, the market is poised for steady growth, with innovation and data-driven care shaping global treatment trends.
Refractory Glaucoma Treatment Market Historical Analysis
The historical analysis of the refractory glaucoma treatment market shows a shift from traditional drug therapies and conventional surgeries toward advanced, technology-driven solutions. Early treatment relied heavily on medications and procedures like trabeculectomy and tube shunts, but rising glaucoma prevalence and recognition of refractory cases increased demand for more effective options. Minimally invasive glaucoma surgeries (MIGS) gained traction in the 2010s due to improved safety and faster recovery, capturing a growing share of the device market. In recent years, sustained-release implants, combination therapies, and digital monitoring tools have emerged, enabling long-term pressure control, better adherence, and personalized care, shaping the modern treatment landscape.
Sources
Primary Research Interviews:
Ophthalmologists specializing in glaucoma
Eye surgeons performing MIGS and drainage device procedures
Optometrists managing advanced glaucoma cases
Medical device manufacturers and R&D teams for glaucoma treatment
Databases:
World Health Organization (WHO) Eye Health Data
International Agency for the Prevention of Blindness (IAPB)
OECD Health Statistics
Global Burden of Disease (GBD) Eye Care Reports
Magazines:
Ophthalmology Times
Review of Ophthalmology
Eye World
MedTech Insight
Retina Today
Journals:
Journal of Glaucoma
Investigative Ophthalmology & Visual Science (IOVS)
American Journal of Ophthalmology
Clinical & Experimental Ophthalmology
Ophthalmology Science
Newspapers:
The New York Times (Health)
The Guardian (Health)
Financial Times (Healthcare)
The Hindu (Health)
Reuters Health
Associations:
World Glaucoma Association (WGA)
American Academy of Ophthalmology (AAO)
International Council of Ophthalmology (ICO)
European Glaucoma Society (EGS)
Glaucoma Research Foundation
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients