Recombinant Therapeutic Antibodies and Proteins Market– A Novel Approach to Treat Rare and Chronic Diseases
Therapeutic proteins are engineered in the laboratory for pharmaceutical use, including non-covalent binders. These proteins are highly effective and serve as modernized treatment for rare as well as chronic diseases. Protein therapeutics offer custom-made treatment approach by supporting a specifically targeted therapeutic process by compensating the deficiency of an essential protein. Recombinant proteins have gained significant traction for therapeutic applications and the number of proteins either launched or approved into clinical trials has continually increased over the past two decades. According to the National Center for Biotechnology Information (NCBI) data of 2017, the U.S. Food and Drug Administration (FDA) approved over 140 recombinant therapeutic proteins for human use and several hundred are currently in development. Majority of these proteins are recombinant monoclonal antibodies.
The global recombinant therapeutic antibodies and proteins market size was valued at US$ 91.2 billion in 2017, and is expected to witness a robust CAGR of 12.2% over the forecast period (2018 – 2026).
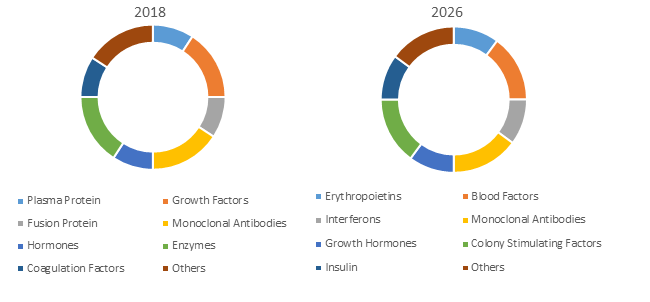
Figure 1. Global Recombinant Therapeutic Antibodies and Proteins Market Share (%), By Drug Class
To learn more about this report, Download Free Sample
Source: Coherent Market Insights Analysis (2018)
Advancements in recombinant therapeutic drugs are expected to bolster the recombinant therapeutic antibodies and proteins market growth
Integration of novel approaches and strategies to modify protein drug products is an important aspect. Advancements in recombinant protein technologies have allowed drug manufacturers and developers to adjust required functional characteristics of proteins of interest while maintaining product efficacy and safety.
Various recombinant technologies are currently in use to increase the half-life, functionality, and targeting of novel therapeutic protein drugs and increase product purity and volume. For instance, protein conjugation and derivatization methods, including albumin-fusion15, PEGylation and Fc-fusion are currently being used to extend a drug’s circulating half-life.
Monoclonal antibodies (mAbs) are most commonly used recombinant therapeutic products. The production of most monoclonal antibody products is easily amenable to effective platform-based approaches as antibodies are highly specific and the risk of unexpected safety issues in human clinical trials of mAb products is less as compared to other types of therapeutic products. The constant evaluation of monoclonal antibody drugs in new and expanded clinical indications results in constant demand for products in clinical studies ensuing increased sales in newly approved indications.
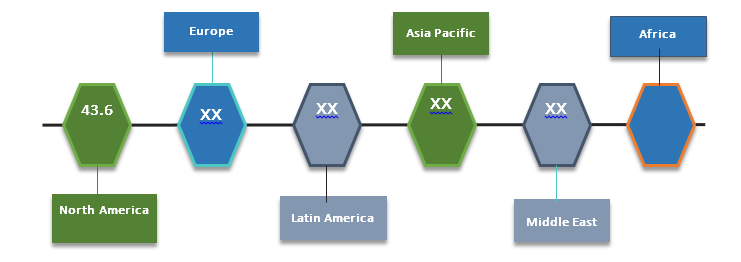
Figure 2. Recombinant Therapeutic Antibodies and Proteins Market (US$ Bn), By Region, 2017
To learn more about this report, Download Free Sample
Source: Coherent Market Insights Analysis (2018)
Increasing government support for novel drug development is expected to augment the market growth
Governments are supporting research and development of novel products due to rapid advancements in biomedical science and technology to address unmet medical needs. In 2012, the Food and Drug Administration Safety and Innovation Act (FDASIA) was signed to provide the FDA with the capability to establish breakthrough therapy designation (BTD), a new program within the Expedited Programs for Serious Conditions. It was designed to be available for drugs designed to treat a serious condition and that have been shown to exhibit initial clinical evidence of significant improvement over existing treatments.
Moreover, increasing number of rare diseases significantly impacting public health is expected to drive growth of the recombinant therapeutic antibodies and proteins market size. According to National institute of Health (NIH) 2018 report, an estimated 7,000 different disorders collectively affect about 10% of the U.S. population, mainly young children and many lack effective treatments. In order to encourage the development of drugs that specifically address unmet medical needs, in 2013, an orphan designation was given for drugs used for the treatment of less than 200,000 patients in the U.S.
However, government regulations are stringent for production and approval of recombinant therapeutic drugs in order to ensure that these products are effective and safe.
Major players operating in the global recombinant therapeutic antibodies and proteins market include Abbott, Amgen Inc., Biogen Inc., Eli Lilly and Company, F. Hoffmann-La Roche, Johnson and Johnson, Merck & Co., Novo Nordisk, Pfizer Inc., and Sanofi S.A.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients