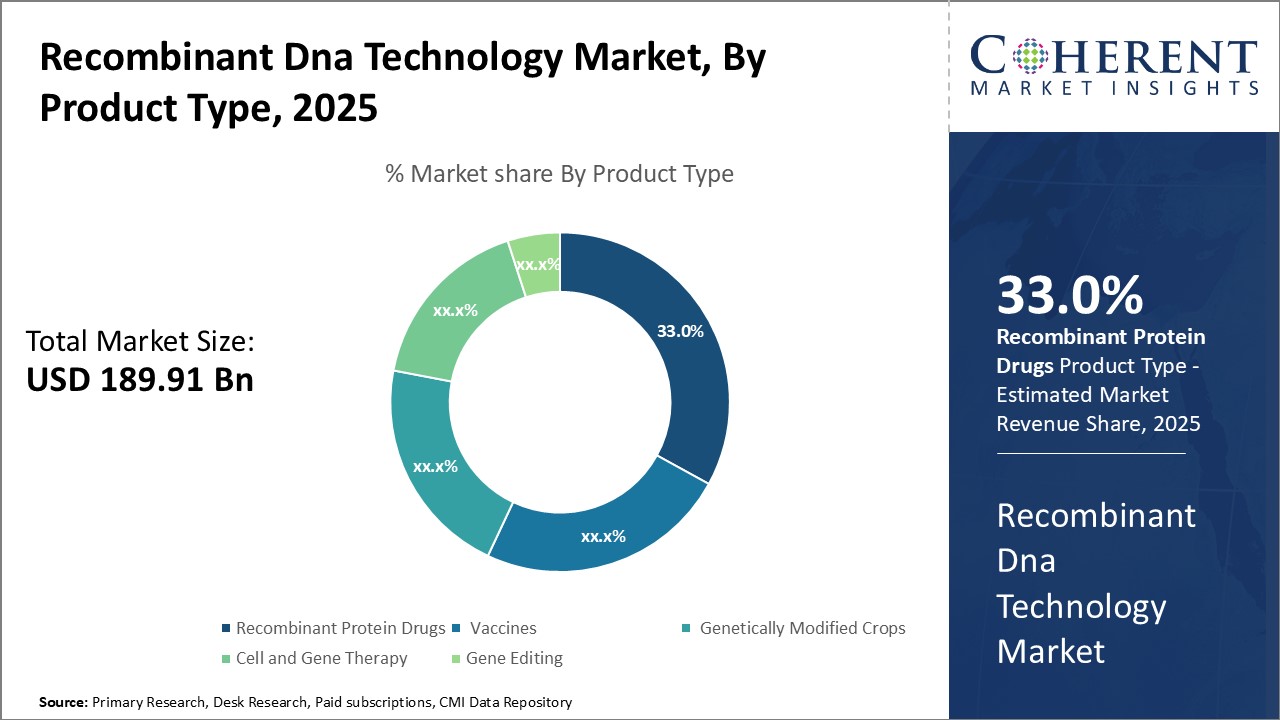
The recombinant DNA technology market is estimated to be valued at USD 189.91 Bn in 2025 and is expected to reach USD 365.62 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 9.8% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The recombinant DNA technology market is expected to witness rapid growth during the forecast period owing to increasing investments in research and development activities by pharmaceutical and biotech companies. With rising demand for protein therapeutics and monoclonal antibodies, the demand for recombinant DNA technology is also rising. Continuous advancements in genetic engineering tools and genomic analysis are further expected to drive the market growth. Adoption of recombinant proteins in healthcare and increasing availability of recombinant vaccines and hormones are some key factors propelling the market growth.
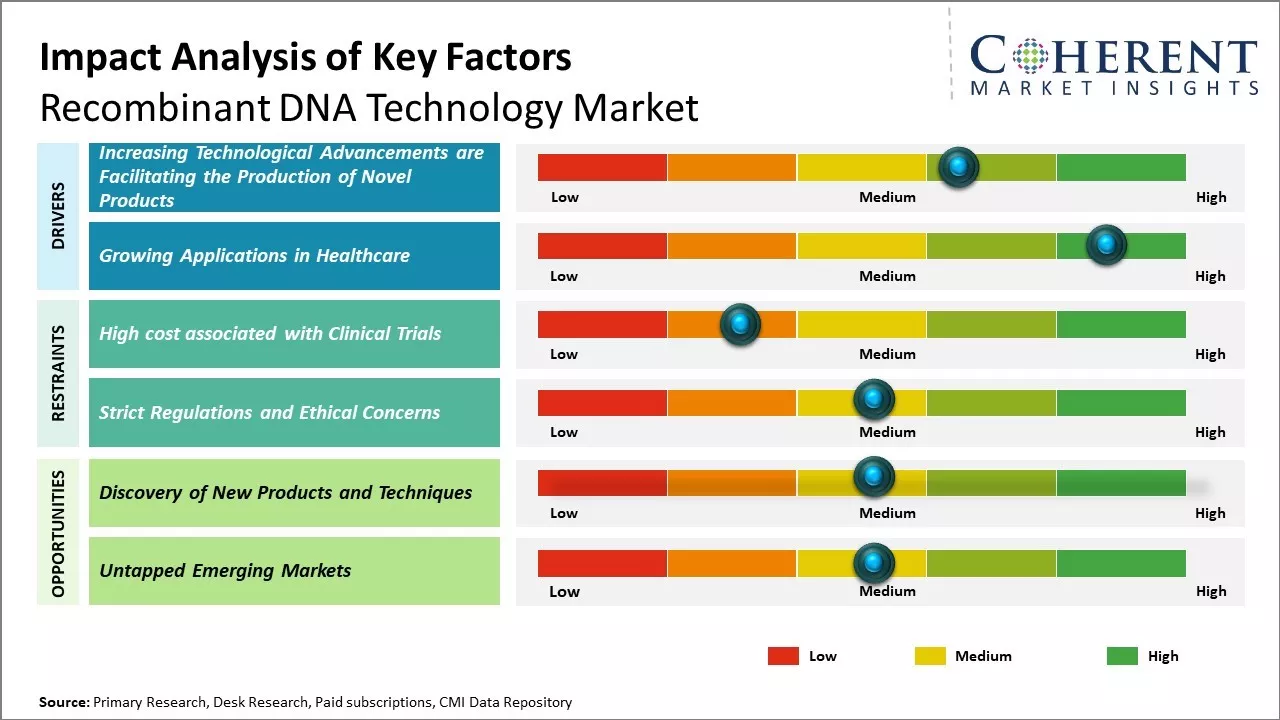
Increasing Technological Advancements are Facilitating the Production of Novel Products
Tremendous advancements combined with a wide range of applications of recombinant DNA technology to treat life-threatening human diseases such as cancer, diabetes, and infectious diseases are expected to be the major factors augmenting the market growth. According to the Global Diabetes Community, in 2021, an estimated 537 million adults are suffering from diabetes worldwide, and the number is expected to escalate to 643 million by 2030. Moreover, in February 2021, according to the World Health Organization (WHO), an estimated 6.7 million deaths were directly caused by diabetes and 2.2 million deaths were attributable to hyperglycemia or high blood glucose levels. Advancements in recombinant technologies have facilitated the development of long-acting therapeutic proteins for drug development as well as introduced gene therapy, which is a novel molecular medicine, thus supporting the growth of the recombinant DNA technology market by creating a positive impact on the treatment of genetic diseases. Moreover, the novel gene-editing tool – Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR), can be used to target the destruction of abnormal genes in human cells, and it has provided scientists a powerful way to make precise changes to DNA of microbes, plants, mice, dogs, and human cells.
Get actionable strategies to beat competition: Download Free Sample
Growing Applications in HealthcareThe field of recombinant DNA technology has experienced significant growth in applications related to healthcare. Many complex and challenging diseases such as cancer, genetic disorders, and infectious diseases are now being addressed through recombinant DNA approaches. By allowing the large-scale production of important biomolecules like insulin, growth hormones, and coagulation factors, this technology has revolutionized the treatment of diabetes, growth deficiencies, and hemophilia. Scientists are also using it to develop novel vaccines, model gene functions, and interactions, conduct gene therapy clinical trials, and better understand the molecular basis of diseases. Going forward, as the technology becomes more advanced and efficient, it is likely to play an even greater role in developing personalized medicine and precision therapies. Many pharmaceutical and biotech companies have increased their R&D investments in this area in search of the next breakthrough treatment. Its ability to produce custom therapeutic proteins on demand position it at the forefront of advancing modern medicine over the coming decade.

To learn more about this report, Download Free Sample
Market Challenges – Strict Regulations and Ethical ConcernsStrict regulations and ethical concerns surrounding recombinant DNA technology are indeed restraining the growth of the global market. Governments across the world are introducing stringent rules and guidelines to ensure the safe development and application of recombinant DNA products. While recombinant DNA has yielded medical breakthroughs in areas such as insulin production and growth hormones, there are ongoing debates around its use in human germline editing. Many scientists argue that hereditary genome editing could have unpredictable consequences and may disadvantage future generations who cannot consent. Considering the associated health and ethical risks, several nations like France, Germany and Hungary have banned human germline modification.
Market Opportunities – Untapped Emerging Markets
Countries such as China are poised for rapid development and many have large, young populations with growing incomes. As living standards increase, the demand for advanced healthcare solutions will rise sharply. Recombinant DNA technology allows for the production of important therapies like insulin at large scale and low costs. This makes such treatments accessible for millions across developing nations for the first time. Countries in Asia Pacific, Africa, and Latin America currently spend the least on healthcare globally. However, their governments are increasingly prioritizing public health and control over deadly diseases. Successful implementation of recombinant DNA based drugs and vaccines targeting critical illnesses can drive immense social impact.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
Insights, By Product Type: Ability to Treat Serious Medical Conditions Drives the Recombinant Protein Drugs SegmentThe product type segment includes recombinant protein drugs, vaccines, genetically modified crops, cell and gene therapy, and gene editing. The recombinant protein drugs sub-segment is estimated to hold 33.0% of the market share in 2025 owing to its ability to treat serious and life-threatening medical conditions. Recombinant protein drugs work by mimicking natural human proteins and replacing missing or defective versions in the body. This allows them to treat genetic disorders, cancers, and other diseases. One of the main drivers of growth for this segment is its use in treating hemophilia. Hemophilia is a rare bleeding disorder caused by missing or defective blood clotting factors. Recombinant versions of these factors, such as recombinant Factor VIII and Factor IX, have revolutionized hemophilia treatment. They provide lifesaving bleed protection and have vastly improved patients' quality of life compared to older therapies derived from blood donations. As such, they are now the standard of care globally.
Insights, By Application: Therapeutics Dominates the Disease Burden
The application segment includes therapeutics, agriculture, research, and others. The therapeutics sub-segment is estimated to hold 58.9% of the market share in 2025 owing to the immense global disease burden. Therapeutics covers the treatment of diseases using biological molecules produced from recombinant DNA technology. This includes blockbuster recombinant protein drugs, as well as viral and bacterial vaccines. A key factor driving the therapeutics segment is the rise of non-communicable diseases (NCDs) like diabetes, cancer, cardiovascular disease, and chronic respiratory illness. NCDs account for over 70% of global deaths per year according to the World Health Organization. Their growing prevalence creates a huge demand for recombinant therapeutics to treat and manage these conditions. Vaccines also represent a major application area. Rising immunization rates around the world mean more people are protected from communicable diseases like influenza, pneumonia, measles, and HPV cancer. This makes vaccines one of the most effective and cost-effective health interventions.
Insights, By End User: Biotechnology and Pharmaceutical Companies Lead Development and Commercialization
The end user segment includes biotechnology and pharmaceutical company, diagnostic laboratories, academic and government institutes, and others. The biotechnology and pharmaceutical company sub-segment is estimated to hold 38.9% of the market share in 2025 due to playing a leading role in research, development and distribution of recombinant products and technologies. These companies drive significant investment into R&D to innovate new recombinant vaccines, biosimilars, cell, and gene therapies targeting major diseases. They employ molecular biologists, genetic engineers, and other scientific experts focused entirely on exploiting the possibilities of recombinant DNA. The leading R&D and commercialization role of biopharma companies therefore cements their position as the top end user driving utilization of recombinant technologies. Their expertise and resources continue powering medical and scientific breakthroughs in this field.
Need a Different Region or Segment? Download Free Sample
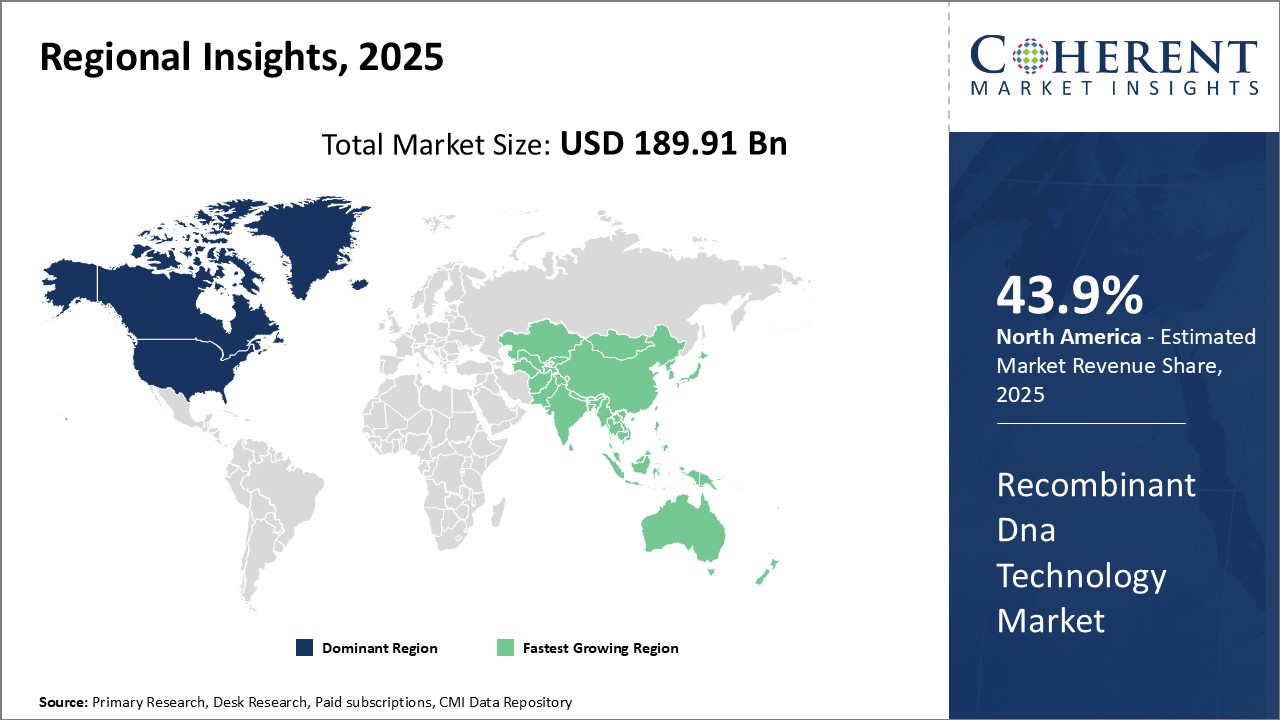
North America remains the dominant region in the global recombinant DNA technology market and is estimated to hold 43.9% of the market share in 2025. This can be attributed to the region's strong foothold in biotechnology research and presence of major industry players. The U.S. alone accounts for the majority of global spending on healthcare research and development activities. This has translated into significant investments in the development and adoption of new techniques like recombinant DNA technology to design novel drugs and medical therapies. Moreover, there is a favorable regulatory environment that promotes innovation through initiatives to speed up approval processes for recombinant products. Many leading biotech companies have their headquarters located in the U.S. and Canada. This industry concentration has helped in the development of strategic collaborations between research institutes and private corporations. Through partnerships, companies are able to leverage each other's expertise to advance their recombinant R&D projects. Additionally, funding and grants from government agencies actively support ongoing research in this field. The strong support for biotechnology from both private and public stakeholders has cemented North America's supremacy in the recombinant technology domain.
Asia Pacific region is emerging as the fastest growing market for recombinant DNA technology. Countries like China and India offer large patient populations and growing healthcare expenditure. This provides lucrative opportunities for companies offering recombinant therapeutics and diagnostics. Local governments also prioritize the development of domestic biotechnology industries as part of the national agenda. For instance, China has launched special economic zones and funding programs to encourage foreign investments and transfer of advanced technologies. The lower manufacturing costs and availability of highly skilled workforce in Asia Pacific is attracting leading North American and European firms to set up regional R&D centers and production facilities in the region. This will help address the needs of local markets more efficiently while also making recombinant solutions more affordable. The favorable government policies and expanding regional capacities indicate strong future potential for the recombinant DNA technology market in Asia Pacific.
Recombinant Dna Technology Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 189.91 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.8% | 2032 Value Projection: | USD 365.62 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
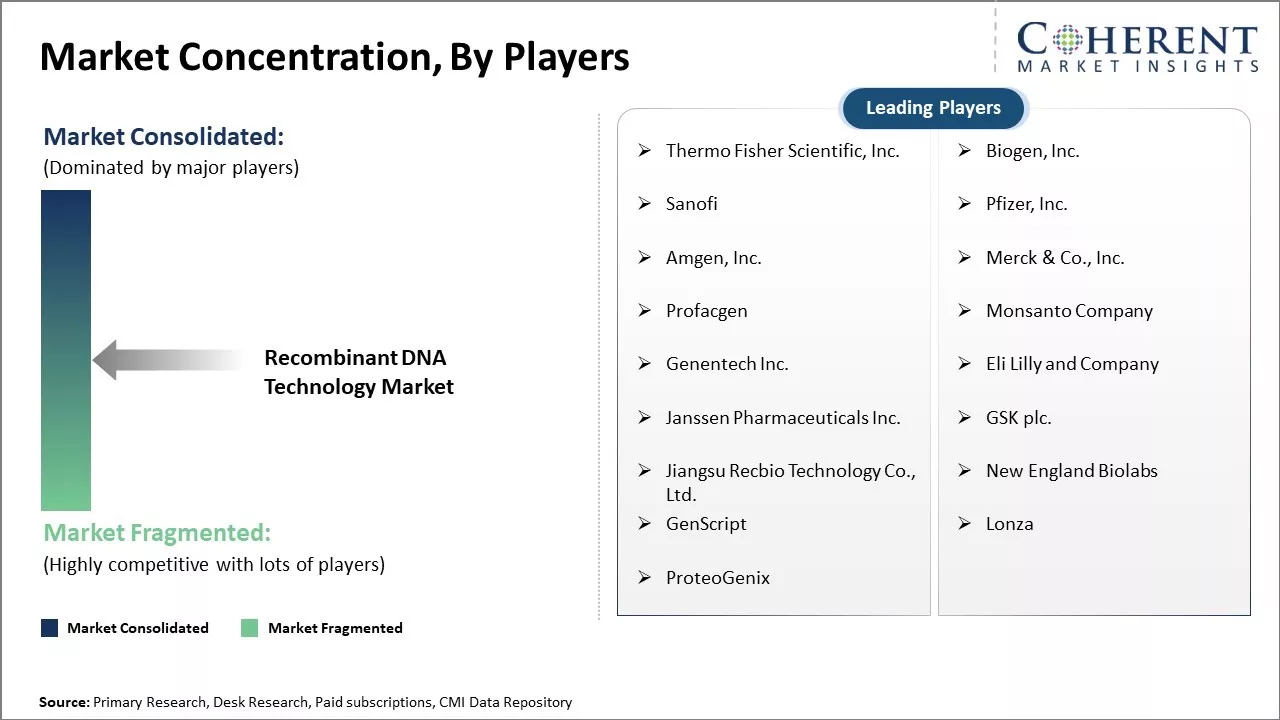
| Companies covered: |
Thermo Fisher Scientific, Inc., Biogen, Inc., Sanofi , Pfizer, Inc., Amgen, Inc., Merck & Co., Inc., Profacgen, Monsanto Company, Genentech Inc., Eli Lilly and Company, Janssen Pharmaceuticals Inc., GSK plc., Jiangsu Recbio Technology Co., Ltd., New England Biolabs, GenScript , Lonza, and ProteoGenix |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients