Global radiopharmaceuticals in nuclear medicine market is estimated to be valued at USD 7.72 Bn in 2025 and is expected to reach USD 13.85 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 8.7% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
Rapid advancements in radiopharmaceutical production and increasing prevalence of cancer and cardiac ailments can drive the market growth during the forecast period.
Rising geriatric population susceptible to chronic diseases and increasing availability of diagnostic imaging modalities can drive the market growth. Furthermore, ongoing investments by key players to develop innovative radiotracers and radiopharmaceuticals can also drive the radiopharmaceuticals in nuclear medicine market growth. However, shorter half-lives of radiotracers posing bottlenecks in distribution and supply chain challenges can hamper the market growth.
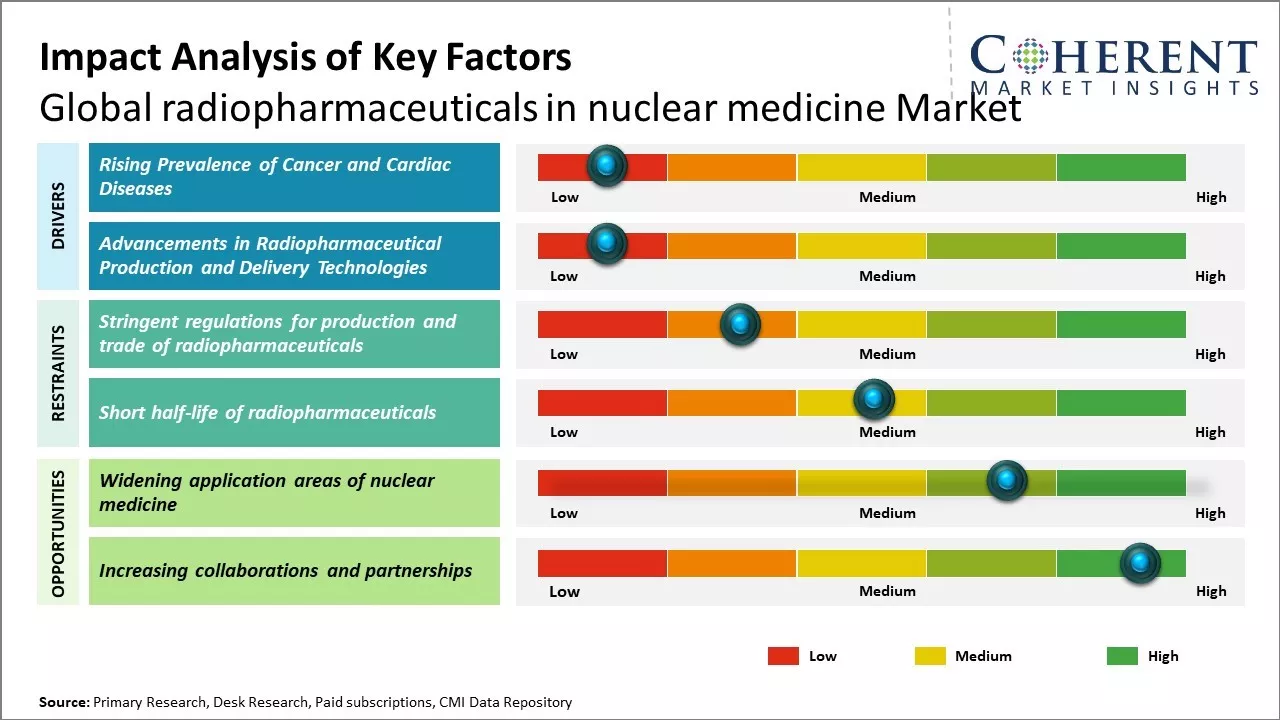
Rising Prevalence of Cancer and Cardiac Diseases
Global radiopharmaceuticals in nuclear medicine market growth is driven by rising prevalence of various cancer and cardiac diseases across the globe. According to estimates, cancer has now become one of the leading causes of mortality worldwide with over 10 million new cases and 6 million deaths reported annually. There has been rise in incidence of cancer due to rapidly aging population, increasing adoption of western lifestyles, and growing exposure to various environmental risk factors like tobacco usage, pollution and radiation. Nuclear medicine plays a vital role in cancer management right from staging and restaging to assessing treatment response. Various radiotracers available have proven useful for imaging tumors and detecting their spread in different parts of the body. Positron emission tomography radiotracers like FDG are commonly used for whole body cancer screening and monitoring therapy progress. There has been rising burden of cardiovascular diseases due to sedentary lifestyles and unhealthy diets. Diseases like coronary artery disease, congenital heart diseases, stroke are leading to millions of deaths each year. Myocardial perfusion imaging using radiotracers is an important non-invasive diagnostic technique for evaluating coronary artery disease by assessing blood flow to the heart muscle. It helps determine the nature and extent of ischemia and influence treatment decisions. This wide application of radiopharmaceuticals for diagnosis and management of these highly prevalent chronic disorders can drive the market growth.
Get actionable strategies to beat competition: Download Free Sample
Advancements in Radiopharmaceutical Production and Delivery Technologies
Advancements in radiopharmaceutical production and delivery technologies can drive the radiopharmaceuticals in the nuclear medicine market growth. With innovation in radiochemistry and automation processes, drug manufacturers are now able to produce more complex radiopharmaceuticals in small batches with high purity levels. This allows for personalized dosing and late-stage customization of radiotracers for enhanced diagnosis of various diseases. For example, Positron Emission Tomography (PET) radiotracers synthesized using module-based synthesis platforms provide flexibility to meet dynamic clinical needs while ensuring consistent quality. With ongoing research in radiobiology, many new targeted radiopharmaceuticals are also in the pipeline for theragnostic applications. Radiopharmaceuticals labeled with alpha-emitters and combined with monoclonal antibodies can be used for treating cancers. Nanoparticle-based drug delivery systems are being developed to achieve precise target localization of radiotracers. For instance, trials show liposome-encapsulated radiotracers can potentially improve outcomes for neuroendocrine tumor imaging and treatment. Such innovations pave way for personalized nuclear medicine approaches. With hybrid PET-MR imaging, physicians can get molecular and anatomical information simultaneously, aiding improved diagnostics. As per the National Cancer Institute, PET-MR scans detected 30% more cancer lesions compared to PET-CT in one 2021 trial involving 300 patients with various cancers. This highlights the transformational role next-gen imaging can play. Constant progress in production methods and delivery mechanisms along with supportive healthcare policies can make nuclear medicine a mainstay for myriad clinical applications in the near future.
Key Takeaways from Analyst
Rising prevalence of cancer and cardiac diseases can boost demand for radiopharmaceuticals that help diagnose and treat such conditions. Growing geriatric population susceptible to chronic illnesses can also drive the market growth. Rapid technological advancements enable development of novel radiotracers with better sensitivity and specificity for specialized diagnostics. North America currently dominates the market, owing to high healthcare expenditures and widespread adoption of nuclear medicine procedures. However, Asia Pacific is predicted to emerge as the fastest growing regional market due to increasing investments towards improving radiopharmaceutical infrastructure and expanding diagnostic capabilities.
Stringent regulations regarding production and handling of radioisotopes can pose challenge to market players and may hamper radiotracer supply on occasion. Short half-lives of various radioisotopes mandate prudent planning for just-in-time manufacturing and distribution to clinical sites. Limited number of indicator applications for certain radiotracers can hamper the market growth. Although ongoing research aims to discover new molecular targets, developmental timelines are often protracted. Despite such restraints, favorable reimbursement policies accommodating nuclear medicine reimbursements can offer potential opportunities for industry stakeholders to enhance penetration in developed markets and boost capacity utilization.
Market Challenges: Stringent regulations for production and trade of radiopharmaceuticals
Stringent regulations for production and trade of radiopharmaceuticals can hamper the radiopharmaceuticals in nuclear medicine market growth. Regulations play a key role in ensuring safety, efficacy and quality standards are maintained for such medically important radioactive substances. However, over-regulation can also stifle innovation and accessibility to these life-saving drugs. Nuclear medicine involves administration of small amounts of radiopharmaceuticals to patients for diagnosis and treatment. These radiopharmaceuticals contain radioactive tracers, which helps medical professionals obtain functional and molecular level information about organs and tissues. Producing and handling radioactive isotopes requires strict oversight to minimize risks to patients, health workers as well as general public. For instance, International Atomic Energy Agency mandates high standards for radiation safety and security across member nations. Non-compliance with such United Nations guidelines leads to disruptions in cross-border trade of radiotracers. National level regulatory pathways in different jurisdictions also vary vastly, which adds to compliances challenges. For new radiotracers, approval process can take 5-7 years on an average before these are available for patient use after rigorous clinical trials. This deters investments in research and development of latest generation radiopharmaceuticals. According to the data published by OECD Nuclear Energy Agency in 2022, U.S. saw only 15 new drug approvals during 2017-2020 as against over 50 approvals in pharmaceuticals sector, indicating regulatory hurdles.
Market Opportunities: Widening application areas of nuclear medicine
The widening array of applications for nuclear medicine techniques can offer huge opportunity for radiopharmaceuticals market growth. Traditionally used for diagnostic imaging procedures like PET and SPECT scans to detect and stage cancer, cardiological and neurological conditions, nuclear medicine is increasingly being leveraged for therapeutic applications. The ability to administer radioactive pharmaceuticals directly to diseased areas allows for targeted treatments with fewer side effects than conventional external beam radiation therapy. This paradigm shift from diagnosis alone to integrated diagnosis and treatment planning will boost demand for radiotracers and radiopharmaceuticals. Emerging applications such as radiotherapy for metastatic bone cancer treatment using radium-223 or lutetium-177 are demonstrating high response rates with manageable toxicity profiles. Their success may lead to approvals for additional cancer types. Combining PET imaging abilities with radiotherapeutic payloads allows for personalized medicine by pinpointing precise tumor locations and verifying treatment effect in vivo. Development of theragnostic agents capable of both imaging and therapy - could revolutionize management of many chronic diseases if ongoing clinical trials prove successful. According to the International Atomic Energy Agency, more than 50 million nuclear medicine procedures are performed globally each year for diagnosis and image-guided treatments. However, only a fraction involves integration of diagnosis and therapy currently due to limitations in available theranostic agents.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
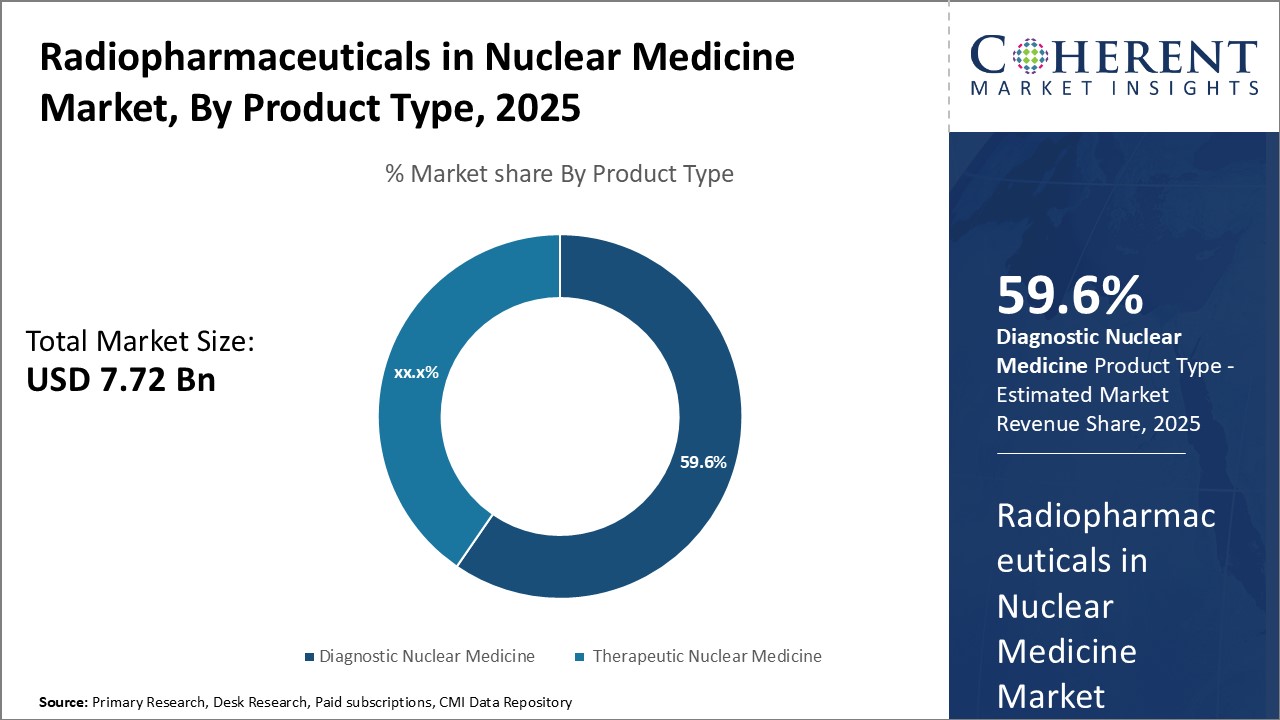
By Product Type - Rising prevalence of chronic diseases
In terms of product type, therapeutic nuclear medicine segment is estimated to contribute the highest market share of 59.6% in 2025, owing to rising prevalence of chronic diseases such as cancer. Therapeutic nuclear medicine involves the use of radioactive pharmaceuticals to treat various conditions. Increasing incidence of cancer worldwide can drive the segment growth. For instance, according to the data from WHO, there were about 19.3 million new cancer cases globally in 2022. Growing geriatric population can also drive the segment growth as older adults are at higher risk of developing cancer and other chronic illnesses. Moreover, ongoing research and development activities focused on expanding therapeutic applications of nuclear medicine further drives the segment growth. New product approvals and pipeline drugs for treating more cancer types and tumors can boost therapeutic nuclear medicine's market share in the near future.
By Application - Growing aging demography
In terms of application, oncology segment is estimated to contribute the highest market share of40.5% in 2025, owing to growing aging demography globally. Since cancer is predominantly a disease of old age, rising senior population directly increases the incidence of various cancer types. Around 60% of all new cancer cases and 70% of all cancer deaths occur in people above 65 years of age. Rapid aging of society due to increasing life expectancy and declining fertility rates worldwide contributes significantly to rising burden of cancer. Technological advancements have enabled improved diagnostics and treatment monitoring of cancer via nuclear medicine techniques like PET scans. This boosts adoption of radiopharmaceuticals in oncology applications.
By End User - Hospital-centric healthcare systems
In terms of end user, hospitals segment is estimated to contribute the highest market share of 42.5% in 2025, owing to hospital-centric nature of most healthcare systems globally. Since nuclear medicine diagnostic and therapeutic procedures require specialized equipment and trained technicians, hospitals are typically the preferred end user setting. These have the necessary infrastructure and expertise to effectively perform complex scans like SPECT and PET imaging. The administration of radiopharmaceuticals for cancer treatment usually takes place in hospitals as in-patient therapies under medical supervision. Infrastructure expansion of hospitals as well as rising hospital admissions and out-patient visits boosts demand for nuclear medicine in hospitals. Their dominance in service provision and reimbursement models make them the primary end users for radiopharmaceuticals.
Need a Different Region or Segment? Download Free Sample
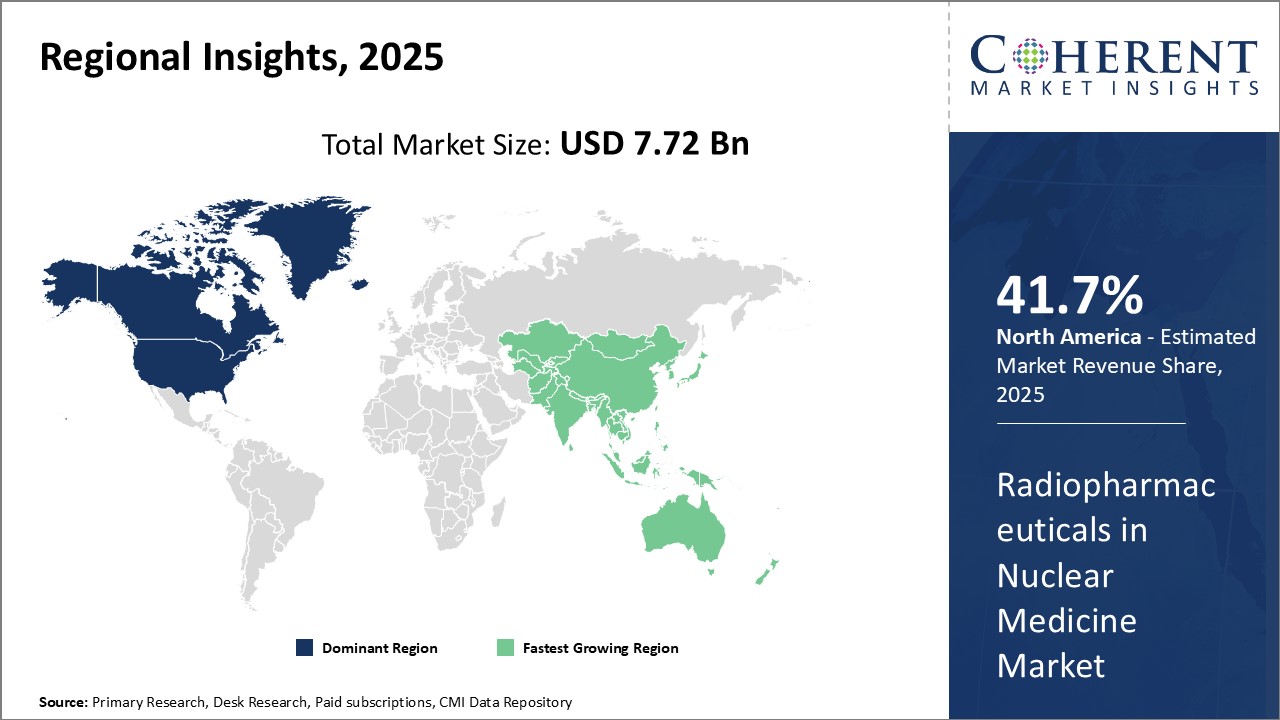
North America dominates the global radiopharmaceuticals market with an estimated market share of 41.7% in 2025. The region has strong presence of leading industry players as well as formidable healthcare infrastructure and research capabilities. Most of the globally recognized players in radiopharmaceuticals manufacturing operate out of the U.S. and have their headquarters located in the region. This allows them to cater to both domestic as well as international markets effectively. The presence of advanced nuclear medicine facilities, availability of sophisticated diagnostic equipment and growing adoption of PET and SPECT scans for clinical diagnosis and treatment monitoring can drive the market growth.
Asia Pacific region is projected to witness the fastest growth. Factors such as rising healthcare expenditure, growing geriatric population, increasing incidence of target diseases and presence of untapped opportunities drives the market growth in the region. China, India and Japan are anticipated to be the major revenue generators. While China and India are focused on providing radiopharmaceuticals at competitive prices, Japan is contributing through its technological innovations. Furthermore, developing healthcare infrastructure, rising medical tourism and favourable regulatory framework are attracting many global players to enter Asia Pacific markets through joint ventures, partnerships and acquisitions. However, lack of skilled professionals and price control regulations in some countries continue to can hamper the market growth.
Radiopharmaceuticals in Nuclear Medicine Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 7.72 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.7% | 2032 Value Projection: | USD 13.85 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
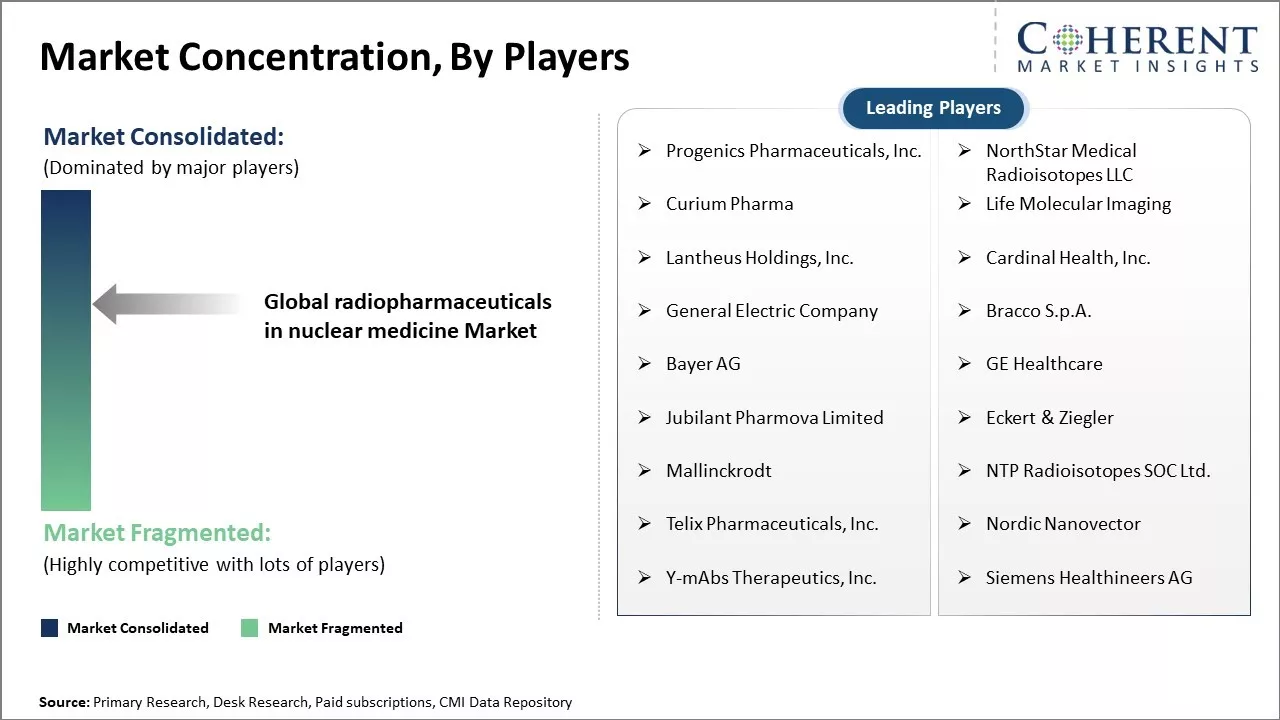
Progenics Pharmaceuticals, Inc., NorthStar Medical Radioisotopes LLC, Curium Pharma, Life Molecular Imaging, Lantheus Holdings, Inc., Cardinal Health, Inc., General Electric Company, Bracco S.p.A., Bayer AG, GE Healthcare, Jubilant Pharmova Limited, Eckert & Ziegler, Mallinckrodt, NTP Radioisotopes SOC Ltd., Telix Pharmaceuticals, Inc., Nordic Nanovector, Y-mAbs Therapeutics, Inc., Siemens Healthineers AG |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
*Definition: Radiopharmaceuticals are pharmaceutical preparations containing radioactive isotopes used as medical tracers in functional imaging techniques such as positron emission tomography (PET) and single-photon emission computed tomography (SPECT). These are typically used for targeted therapy and diagnostic procedures in nuclear medicine. Radiopharmaceuticals consist of a radioisotope complex or compound attached to a targeting molecule such as a protein, antibody or chemical agent to direct it to a specific organ or lesion in the body. Once administered, radiopharmaceuticals allow physicians to non-invasively image and assess physiological or pathological conditions in vivo at a cellular or molecular level.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients