Pulmonary surfactant is a complex mixture of phospholipids and proteins that creates a unique interface separating alveolar gas and liquids at the alveolar cell surface, reducing surface tension, and maintaining lung volumes at end expiration. Reduction of the surface tension at the air–liquid interface is a requirement for respiratory function following birth. Deficiency of pulmonary surfactant causes respiratory failure in premature infants, or infantile respiratory distress syndrome (IRDS). The adequacy of pulmonary surfactant is maintained by unique and highly regulated systems mediating the synthesis, secretion, reutilization, and catabolism of surfactant. Loss or inactivation of pulmonary surfactant later in life occurs in the adult respiratory distress syndrome (ARDS), a significant cause of morbidity and mortality following infection, shock, or trauma. Mutations in genes regulating surfactant homeostasis, including SFTPA, SFTPB, SFTPC, ABCA3, TITF1, and CSF2RA cause acute and/or chronic lung disease in newborn infants, children, and adults. Disorders of GM-CSF signaling inhibit surfactant lipid and protein catabolism by alveolar macrophage causing pulmonary alveolar proteinosis (PAP).
Global pulmonary surfactant market size was valued at US$ 578.7 million in 2022 and is expected to witness a CAGR of 4.50 % over the forecast period (2022 – 2030).
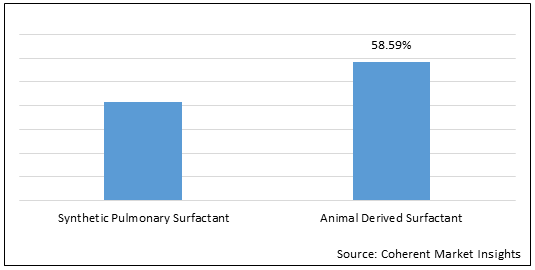
Figure 1.Global Pulmonary Surfactant Market Share (%), by Type, 2022
To learn more about this report, Download Free Sample
Pulmonary Surfactant Market-Drivers
Increasing preference of animal derived surfactant for the treatment of respiratory distress syndrome is driving the pulmonary surfactant market growth over the forecast period.
Increasing preference of animal derived surfactant for the treatment of respiratory distress syndrome is driving the pulmonary surfactant market growth over the forecast period. For instance, in May 2021, according to the article published in Archives of Clinical and Medical Case Reports, an open access, peer reviewed Journal, Poractant alfa (Curosurf), significantly reduced the rates of pneumothorax (the presence of air or gas in the cavity between the lungs and the chest wall, causing collapse of the lung) in preterm infants with respiratory distress syndrome. This reduction was more significant in VLBW (very low birth weight) than non-VLBW (very low birth weight) infants.
Furthermore, on April 11, 2022, an article was published in National Center for Biotechnology Information, a branch of the National Institutes of Health, which reported that when animal derived pulmonary surfactant is combined with budesonide, a corticosteroid or steroid, in the treatment of neonatal respiratory distress syndrome can effectively shorten the hospital stay and reduce the time of invasive mechanical ventilation and the incidence of BPD (bronchopulmonary dysplasia). Meanwhile, it does not increase the risk of related complications or death. This approach can be applied clinically.
Pulmonary Surfactant Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2021 | Market Size in 2022: | US$ 578.7 Mn |
| Historical Data for: | 2017 to 2020 | Forecast Period: | 2022 to 2030 |
| Forecast Period 2022 to 2030 CAGR: | 4.50% | 2030 Value Projection: | US$ 822.9 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Boehinger Ingelheim, AbbVie Inc, Chiesi Farmaceutici, ONY Biotech Inc., Lyomark Pharma, Abbott, Aviva Systems Biology Corporation, Windtree Therapeutics, Inc, Tekzima (Noargen), Biomatik, Nanjing Norris Pharm Technology, Reddot Biotech |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
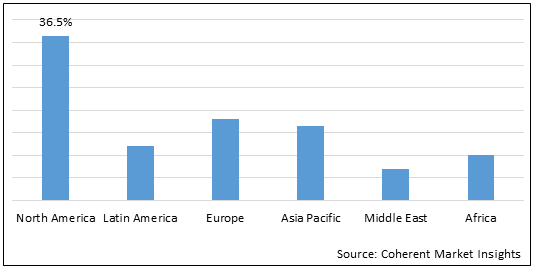
Figure 2.Global Pulmonary Surfactant Market Share (%), by Region, 2022
To learn more about this report, Download Free Sample
Increasing prevalence of respiratory infections are attributing to the highest share of North America market in the global pulmonary surfactant market.
Increasing prevalence of the respiratory infections in the U.S. is expected to drive market growth over the forecast period. For instance, in June 2021, The Centers for Disease Control and Prevention, a national public health agency of the U.S. stated that each year in the U.S., RSV (Respiratory Syncytial Virus) leads to on average approximately 58,000 hospitalizations with 100-500 deaths among children younger than 5 years old and 177,000 hospitalizations with 14,000 deaths among adults aged 65 years or older. Infants, young children, and older adults with chronic medical conditions are at risk of severe disease from RSV infection
Global Pulmonary Surfactant Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease has spread to over 100 countries across the globe and the World Health Organization had declared it a public health emergency on January 30, 2020.The sudden outbreak of COVID-19 has bought the world to a standstill.
Increasing research and development between the key market players of pulmonary surfactant market during COVID-19 pandemic is expected to drive the market growth over the forecast period. For instance, on March 22, 2022, Windtree Therapeutics, Inc., a biotechnology company multiple late-stage interventions for acute cardiovascular and pulmonary disorders, announced results from its Phase 2 study of lucinactant (KL4 surfactant) for patients with severe COVID-19 associated acute respiratory distress syndrome (ARDS) and lung injury. The objective for the study was that the SARS-CoV-2 virus causing COVID-19 uses the angiotensin-converting enzyme 2 (ACE2) receptor for entry into host cells. The Phase 2 trial was designed to assess feasibility, safety, and tolerability of administration of reconstituted lyophilized lucinactant in these critically ill patients.
Global Pulmonary Surfactant Market: Key Developments
Pulmonary Surfactant Market-Restraints
Increasing number of side effect associated with the pulmonary surfactant drug restrain the growth of pulmonary surfactants market. The drugs used in the treatment of respiratory disorders have several side effects. For instance according to a report published by RxList Inc, an online medical resource offering detailed and current pharmaceutical information on brand and generic drugs, on March 3, 2022, common side effects of Surfaxin, a liquid medication used to treat infant respiratory distress syndrome, are related to its administration down a premature infant's breathing tube (endotracheal tube) and include endotracheal tube reflux, skin paleness, endotracheal tube obstruction, and need for dose ductus arteriosus and lung problems.
Key Players
Key players operating in the global pulmonary surfactant market include Boehinger Ingelheim, AbbVie Inc, Chiesi Farmaceutici, ONY Biotech Inc., Lyomark Pharma, Abbott, Aviva Systems Biology Corporation, Windtree Therapeutics, Inc, Tekzima (Noargen), Biomatik, Nanjing Norris Pharm Technology, Reddot Biotech
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients