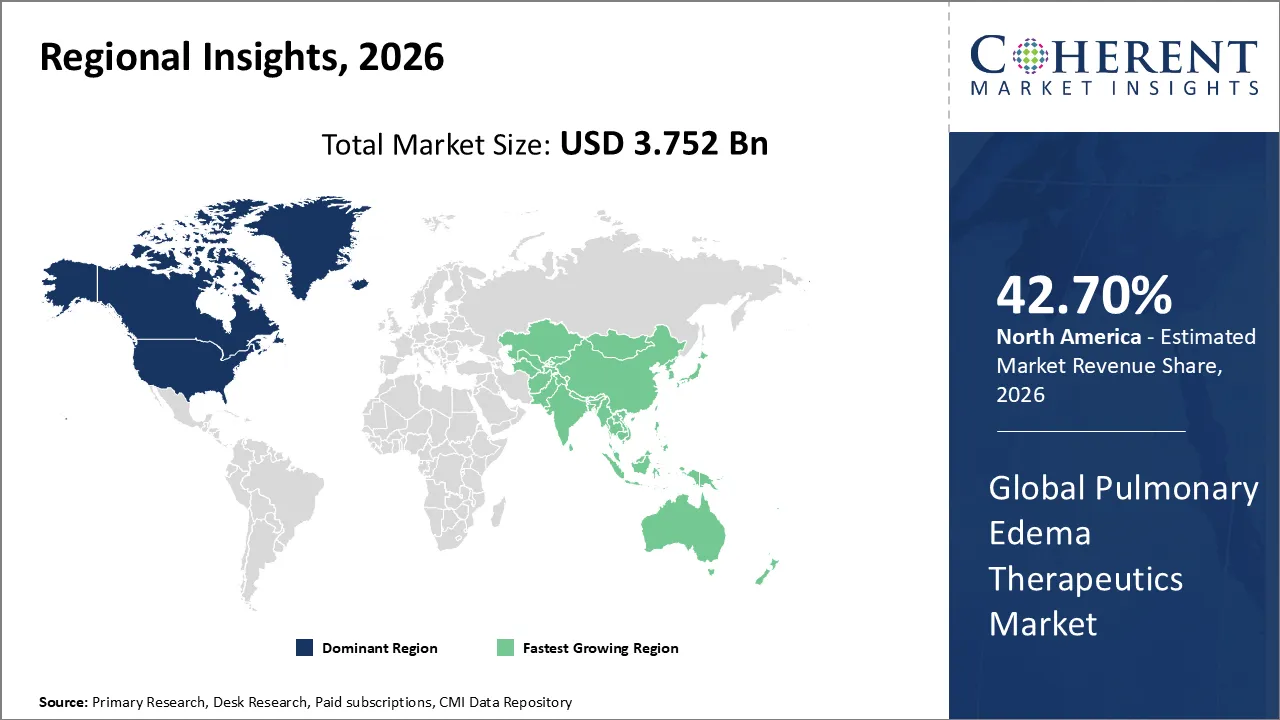
Pulmonary Edema Therapeutics Market is estimated to be valued at USD 3.752 Bn in 2026 and is expected to reach USD 4.690 Bn in 2033, exhibiting a compound annual growth rate (CAGR) of 3.2% from 2026 to 2033.
Pulmonary edema is a condition caused by an accumulation of fluid in the lungs. This fluid accumulates in the lungs' numerous air sacs, making breathing difficult. Pulmonary edema is classified into two types based on where the accumulation of fluid begins in our body. Cardiogenic pulmonary edema occurs, when it is caused by a heart problem such as heart failure. A heart failure usually causes edema in the lungs. Non-cardiogenic pulmonary edema is caused by non-heart conditions such as acute respiratory distress syndrome (ARDS) or high-altitude pulmonary edema (HAPE). Pulmonary edema can sometimes be caused by both heart as well as a non-heart conditions.
The first line of treatment for acute pulmonary edema include supplemental oxygen, which is typically administered through an nasal cannula, a flexible plastic tube with two openings that deliver oxygen to each nostril. Depending on the extent of the condition and the cause of the pulmonary edema, some medications such as diuretics, blood pressure medications, morphine, or inotropes may be prescribed.
|
Current Event |
Description and its Impact |
|
Aging Population Demographics and Healthcare Burden |
|
|
Regulatory Landscape Evolution and Drug Approval Processes |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In terms of drug class, the diuretics segment is expected to lead the market with 42.8% share in 2026. They remain the first line of treatment as they swiftly decrease fluid overload and ease symptoms. Loop diuretics like furosemide are the most common type of diuretic and are used to treat both acute and chronic conditions. This keeps them at the top of the market.
For instance, in September 2025, the FDA approved bumetanide nasal spray, a loop diuretic, to treat swelling caused by heart failure, liver disease, and kidney disease. This new way of giving the drug opens up new treatment options. It strengthens the role of diuretics in treating pulmonary edema by making sure that fluids are quickly removed and patients are more likely to follow their treatment plans in acute care.
In terms of type, the cardiogenic pulmonary edema segment is expected to hold 63.12% share in 2026. Its dominance is due to the fact that heart failure and other cardiovascular diseases have become increasingly common around the world. As the number of older people grows, cardiogenic cases outnumber non-cardiogenic ones. This makes this group the main source of therapeutic demand and market growth. According to the Heart Failure Society of America, around 6.7 million Americans over the age of 20 have HF. By 2030, that number is expected to rise to 8.7 million, then 10.3 million in 2040, and finally 11.4 million by 2050.
For instance, in Septmber 2025, The FDA's approval of Corstasis bumetanide nasal spray, which is a loop diuretic, is important for treating cardiogenic pulmonary edema. Heart failure causes cardiogenic pulmonary edema, and diuretics are the first line of treatment. This new nasal route effectively lowers fluid levels, makes it easier to take medicine, and opens up more treatment options besides oral and intravenous administration.
In terms of route of administration, the intravenous administration segment is projected to account for 71.84% share in 2026. Acute pulmonary edema necessitates prompt intervention, and intravenous administration guarantees swift pharmacological efficacy. Intravenous administration of diuretics, vasodilators, and inotropes is the standard in hospitals, making this the best way to handle emergencies and critical care.
For instance, the FDA's approval of the furosemide autoinjector sNDA is important for treating pulmonary edema. Furosemide is a loop diuretic that is essential for quickly getting rid of fluids. Like intravenous administration, the injectable route works quickly in cases of acute heart failure or kidney disease. This makes emergency treatment options stronger and improves patient outcomes.
In terms of distribution channel, the hospital pharmacies segment is projected to capture 53.09% share in 2026. Pulmonary edema is often a medical emergency that needs immediate treatment and hospitalization. Most therapies are given in inpatient or critical care units, so hospital pharmacies still remain the main way to get them, ahead of retail and online options.
To learn more about this report, Download Free Sample
North America is dominating in the pulmonary edema therapeutics market with 42.7% share in 2026, as more individuals are getting heart and kidney disease, the population is getting older, hospitals are getting better, and new diuretics are being used more quickly. Strong investments in research and development, FDA approvals, and a high demand for acute care all contribute to significant market growth.
For instance, in March 2025, The FDA added to the label for furosemide injection in 2025 stating that it could also be used to treat swelling in people with chronic kidney disease. This makes it even more important in hospital pharmacies, where injectable diuretics are essential for treating pulmonary edema. The approval opens up more treatment options, supports acute care, and strengthens North America's position as the market leader.
Asia Pacific is expected to exhibit the fastest growth with 23.4% share in 2026, due to increasing numbers of individuals are getting heart and kidney disease, there are an extensive number of patients, and cities are getting larger. Rapid adoption of diuretics and advanced therapies is driving strong market growth. This is due to the expansion of hospital infrastructure, increased awareness of acute respiratory care, and government investments in healthcare.
In 2026, the U.S. are going to require more medicines to treat pulmonary edema as the population is getting older, heart failure is becoming more common, and there is an urgent need for quick fluid management. The adoption of these technologies is driven by improvements in minimally invasive delivery systems, more access to hospitals, and changing clinical guidelines. This leads to steady market growth and better outcomes for patients.
For instance, in December 2025, the U.S. FDA accepted review of MannKind’s supplemental new drug application for FUROSCIX ReadyFlow™ Autoinjector, delivering subcutaneous furosemide to treat edema in adults with chronic heart failure or kidney disease. This innovation offers rapid, convenient diuretic therapy outside hospitals, directly addressing pulmonary edema management through improved fluid control.
The market for pulmonary edema drugs in China is expected to expand in 2026 as greater numbers of individuals are getting heart and lung diseases, the population is getting older, and the healthcare system is getting larger. Demand is driven by new technology in diuretic delivery systems and easier access to hospitals. The market is expected to grow steadily at a strong CAGR.
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2025 | Market Size in 2026: | USD 3.752 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2026 To 2033 |
| Forecast Period 2026 to 2033 CAGR: | 3.2% | 2033 Value Projection: | USD 4.690 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Novartis International AG, Cytokinetics Inc., Amgen Inc., AstraZeneca plc., Celularity Inc., and United Therapeutics Corporation. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Epidemiological trends highlight rising cardiovascular disease, respiratory disorders, and aging populations, significantly increasing pulmonary edema incidence. These things make the patient pool larger and push new ideas regarding how to deliver diuretics. As a result, the market for pulmonary edema therapeutics is steadily growing as there is a greater need for quick, effective treatments to manage fluid overload and improve outcomes.
Wearable subcutaneous infusors, intranasal diuretic delivery, and AI-enabled monitoring are just a few examples of how technology is changing how we treat pulmonary edema. These new ideas make it easier for patients to follow their treatment plans, cut down on hospital stays, and speed up the removal of fluids. As adoption rises, the pulmonary edema therapeutics market share expands, reflecting strong demand for modern, efficient, and patient‑centric treatment solutions in 2026.
New ways to deliver drugs, like wearable subcutaneous infusors, intranasal diuretics, and AI-enabled monitoring, are changing the way pulmonary edema is treated. These technologies make it easier to follow the rules, reduce down on hospitalizations, and allow care to be given at home. As adoption accelerates, the pulmonary edema therapeutics market forecast projects strong growth, driven by patient‑centric solutions and expanding global healthcare infrastructure.
The pulmonary edema therapeutics market value is growing steadily as there are so many people with heart and lung problems that cause fluid to build up in the lungs. People with pulmonary edema, which is when an accumulation of fluid makes it hard for the lungs to exchange gases, are often treated with diuretics, vasodilators, and oxygen therapy. In severe cases, they may also need advanced mechanical ventilation. Diuretics remain the principal therapeutic agents owing to their efficacy in alleviating fluid overload. As timely interventions have a direct effect on patient outcomes and treatment pathways, diagnostic improvements and early detection protocols are becoming increasingly important.
Hospitals and specialty clinics continue to be the best places to treat pulmonary edema. This is due to improvements in critical care infrastructure and respiratory support technologies. More individuals can get treatment as there are more outpatient care and home healthcare services available, especially for chronic or recurring cases. Demand patterns in North America show that the market is still growing, due to an aging population and a high rate of heart failure. In Asia-Pacific, new healthcare networks are helping to spread the use of therapies.
Innovations like personalized medicine, therapies guided by biomarkers, and inhalation delivery systems are becoming increasingly commonplace. They could make treatments more effective and reduce systemic side effects. Combining telemedicine and remote monitoring tools can also help with ongoing care and lower the number of acute episodes and hospital readmissions. The market is growing due to clinical needs, new technologies, and a wider range of treatments that all aim to improve patient care.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients