Pulmonary Drug Delivery Systems Market is estimated to be valued at USD 37.57 Bn in 2025 and is expected to reach USD 51.47 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 4.6% from 2025 to 2032.
Analysts’ Views on Global Pulmonary Drug Delivery Systems Market:
Increasing collaboration by key market players to developed digital platform for asthma and chronic obstructive pulmonary disease (COPD) is expected to drive the growth of the market over the forecast period. For instance, in April 2020, AptarGroup, Inc., a provider of consumer dispensing, active packaging, and drug delivery systems and services, and Sonmol, a Chinese digital respiratory therapeutics company, has announced a collaboration for developing a digital therapies and service platform targeting respiratory and other diseases. This collaboration was initially focus on bringing together connected drug delivery devices and the digital platform for asthma and chronic obstructive pulmonary disease (COPD). AptarGroup, Inc., and Sonmol was worked together on expanding Sonmol’s platform services to enable remote patient monitoring, improve patient and physician interactions.
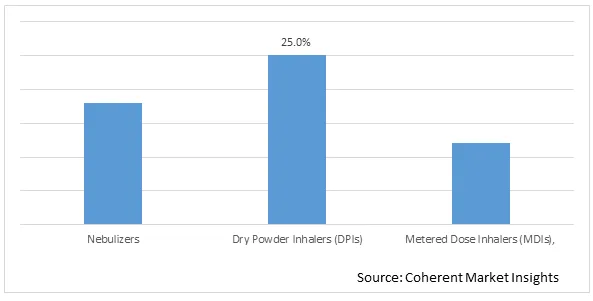
Figure 1. Global Pulmonary Drug Delivery Systems Market Share (%), by Product Type, 2025
To learn more about this report, Download Free Sample
Global Pulmonary Drug Delivery Systems Market– Driver
Increasing prevalance of chronic disorder
Increasing changes in lifestyles is considered a major cause of various chronic disorders, such as diabetes, functional gastrointestinal disorder, eczema, arthritis, asthma, cancer, chronic obstructive pulmonary disease, and others. Thus, the increasing prevalence of chronic disorders globally is expected to boost the growth of the market over the forecast period. For instance, according to the data published by September 2022, the Forum of International Respiratory Societies (FIRS), Global Impact of Respiratory Disease report, an estimated 200 million people has COPD, of which about 3.2 million die each year, making it the third-leading cause of death worldwide, moreover, more than 2.2 million new cases of lung cancer in 2020 and 1.80 million deaths, globally, lung cancer is responsible for 1 in 4 cancer death.
Increasing products launches by the market players
Increasing number of product launches by the market players due to numerous advantages associated with the pulmonary drug delivery systems is expected to foster the market growth over the forecast period. For instance, in July 2020, Teva Pharmaceutical Industries Ltd., a pharmaceutical company, announced that it has launched a bluetooth-enabled version of its ProAir albuterol sulfate rescue inhaler. The ProAir Digihaler’s sensors connect to a companion mobile app to provide inhaler-use information to healthcare providers. The new inhaler was available by prescription to U.S. patients age 4 and older.
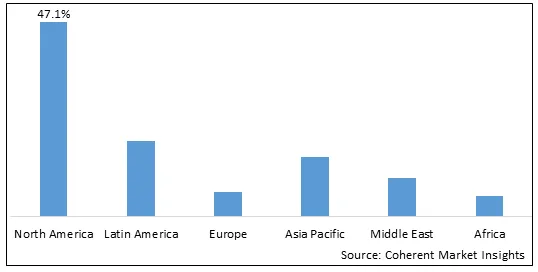
Figure 2. Global Pulmonary Drug Delivery Systems Market Share (%), by Region, 2025
To learn more about this report, Download Free Sample
Global Pulmonary Drug Delivery Systems Market- Regional Analysis
Among regions, North America is estimated to hold a dominant position in the global pulmonary drug delivery systems marketover the forecast period. This is attributed to the increasing product launch by market players. For instance, in October 2025, Aptar Digital Health, part of Aptar Pharma, a global leader in drug delivery and active material science solutions and services, announced a strategic partnership with the Chiesi Group, the international research-focused biopharmaceutical and healthcare group, to bring to market a disease management platform for asthma and chronic obstructive pulmonary disease (COPD)
Global Pulmonary Drug Delivery Systems Market– Impact of Coronavirus (COVID-19) Pandemic
Since the COVID-19 virus outbreak in December 2019, the disease spread to over 100 countries across the globe, and the World Health Organization declared it a public health emergency on January 30, 2020.
The COVID-19 pandemic had a positive impact on the global pulmonary drug delivery systems market, owing to increasing use of metered dose inhaler (pMDI) for patients around the world suffering from chronic respiratory diseases such as asthma, COPD, Cystic Fibrosis and other respiratory conditions caused by COVID-19. Thus market players focused on product launch. For instance, in February 2022, Aptar Pharma, a global leader in drug delivery systems, services and active material science solutions, announced the launch of HeroTracker Sense, a novel digital respiratory health solution that transforms a standard metered dose inhaler (pMDI) into a smart connected healthcare device. HeroTracker Sense is a next generation metered-dose inhaler (MDI) add-on connected device, designed to address patient inhalation technique and adherence.
Pulmonary Drug Delivery Systems Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 37.57 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.6% | 2032 Value Projection: | USD 51.47 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
3M Health Care, Allied Healthcare Products, Inc., AstraZeneca, Boehringer Ingelheim International GmbH., CareFusion Corporation, GF Health Products, Inc., GlaxoSmithKline plc, Merck & Co., Inc., Novartis AG, Omron Healthcare, Inc., Glenmark Pharmaceuticals, PARI Respiratory Equipment, Inc., Koninklijke Philips N.V. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Pulmonary Drug Delivery Systems Market Segmentation:
The global pulmonary drug delivery systems market report is segmented into product type, application, distribution channel, and region.
Based on Product type, the global pulmonary drug delivery systems market is segmented into Nebulizers, Dry Powder Inhalers (DPIs), and Metered Dose Inhalers (MDIs). Out of which, Dry Powder Inhalers (DPIs) segment is expected to dominate the global pulmonary drug delivery systems market during the forecast period and this is due to increasing adoption of various growth strategies such as product approval, partnership, and others by the market players.
Based on Application, the global pulmonary drug delivery systems market is segmented into Asthma, Chronic Obstructive Pulmonary Disease (COPD), Cystic Fibrosis, Allergic Rhinitis, and Others. Out of which, Chronic Obstructive Pulmonary Disease (COPD), segment is expected to dominate the global pulmonary drug delivery systems market during the forecast period, owing to increasing inorganic growth strategies such as acquisition by key market players
Based on Distribution Channel, the global pulmonary drug delivery systems market is segmented into Hospitals Pharmacies, Retail Pharmacies, and Online Pharmacies. The hospital pharamcies segment is expected to dominate the market over the forecast period and this is due to the increasing number of hospital pharmacies globally.
Among all the segmentations, the product type segment has the highest potential due to the increasing product approval by regulatory authorities. For instance, in October 2020, Insmed Incorporated, a biopharmaceutical company, announced that the European Commission (EC) has granted marketing authorization for ARIKAYCE Liposomal 590 mg Nebuliser Dispersion ("ARIKAYCE") for the treatment of nontuberculous mycobacterial (NTM) lung infections caused by Mycobacterium avium complex (MAC) in adults with limited treatment options who do not have cystic fibrosis. Consideration should be given to official guidance on the appropriate use of antibacterial agents.
Global Pulmonary Drug Delivery Systems Market Cross Sectional Analysis:
Increasing inorganic growth strategies such as partnership between the key market players are driving the growth of the product type segment in Europe region. For instance, in December 2020, The Technology Partnership (TTP) plc, a leading independent technology and product development company, announced a partnership with Hovione, a Contract Development and Manufacturing Company to design a new respiratory device; the PowdAir Plus Dry Powder Inhaler (DPI). The innovative dry powder inhaler is designed to be sustainable, efficient, and cost-effective, helping thousands of global asthma and COPD sufferers better access medication at a fraction of the cost.
Global Pulmonary Drug Delivery Systems Market: Key Developments
On May 3, 2023, Hovione, the specialist integrated CDMO, leader in spray drying and particle engineering, and H&T Presspart, leading manufacturer of drug delivery devices, has entered in a strategic partnership to advance the development of Presspart's Sunriser Capsule-based Dry Powder Inhaler platform. The demand for inhalable drugs that require higher drug loads and the delivery of cohesive materials has increased, making it necessary to develop more efficient solutions. To meet this demand, the two companies will work together to develop the Sunriser Dry Powder Inhaler. This innovative and best-in-class capsule-based platform is flexible enough to address both the challenges of classic carrier-based and spray-dried engineered formulations.
On April 3, 2023, PureIMS, a clinical stage pharmaceutical company, announced it has secured a new investment round by Boost-UP Foundation, a private independent foundations. With the current investments the company also pursues opportunities to further enthuse pharma/biotech companies for early-clinical endeavors looking for innovative pulmonary delivery options to develop small and large molecules for local and systemic indications.
On March 16, 2023, Stevanato Group S.p.A., a leading global provider of drug containment, drug delivery, and diagnostic solutions to the pharmaceutical, biotechnology, announced a collaboration with Recipharm, leading Contract Development and Manufacturing Organisation (CDMO). Under the agreement, Stevanato Group will lend its manufacturing experience to support the development and production of pre-fillable syringes for use in Recipharm’s soft mist inhalers. As part of this collaboration, Stevanato Group will provide and manufacture its glass pre-fillable syringe Alba assembled with the Integrated Spray Module (ISMTM) of Recipharm’s soft mist inhaler technology, the Pre-Filled Syringe Inhaler (PFSITM).
Global Pulmonary Drug Delivery Systems Market: Key Trends
Increasing acquisition by the market players
Increasing inorganic growth strategies such as acquisition by the market plaers is expected to drive the growth of the market over the forecast period. For instance, in March 2022, Sandoz, a Novartis AG division, announced that it has successfully acquired Coalesce Product Development Limited., the U.K.-based medical and drug delivery device development company. Through this deal, Sandoz has acquired the significant capabilities and assets of Coalesce, which help it build on its existing portfolio of respiratory medicines and further improve patient access to these high-quality, and complex therapies.
Global Pulmonary Drug Delivery Systems Market: Restraints
Stringent regulatory scenario
Stringent regulatory scenario is expected to hinder growth of the market. Regulatory requirements for pulmonary drug delivery systems are quite challenging as MDIs and DPIs are considered as drug-device combination products, and therefore, are regulated under new provisions by the drug regulatory authorities due to the following factors:
Moreover, various mechanical, chemical, and immunological barriers along with behavioral barriers of poor adherence and poor inhaler technique also limit growth of the market.
The stringent rule and regulation can affect the market growth but if manufacturer follow the rule and regulation during manufacturing of product it helps to maintain the quality of the product thus it is necessary to follow the rules and regulation during manufacturing.
Global Pulmonary Drug Delivery Systems Market- Key Players
Major players operating in the global pulmonary drug delivery systems market include 3M Health Care, Allied Healthcare Products, Inc., AstraZeneca, Boehringer Ingelheim International GmbH., CareFusion Corporation, GF Health Products, Inc., GlaxoSmithKline plc, Merck & Co., Inc., Novartis AG, Omron Healthcare, Inc., Glenmark Pharmaceuticals, PARI Respiratory Equipment, Inc., and Koninklijke Philips N.V.
*Definition: Pulmonary drug delivery is primarily used to treat conditions of the airways, delivering locally acting drugs directly to its site of action. Pulmonary drug delivery offers targeted therapy for the treatment of respiratory diseases, such as asthma, lung cancer, and chronic obstructive pulmonary diseases. Pulmonary drug delivery refers to the systems aimed at targeting the delivery of aerosols directly to epithelial cells and respiratory epithelium by means of inhalation. Based on the desired drug release characteristics, the design of the aerosol formulation is varied to provide prolonged retention or rapid absorption. This pulmonary delivery system can be categorized into immediate-release, controlled-release, and sustained-release systems. These systems vary in its polymeric composition and the excipients. The controlled- and sustained-release pulmonary systems are designed to have advantages like a reduced drug dose, improved therapeutic efficiency, enhanced patient compatibility, quick onset of action, bypassing the hepatic metabolism, localized delivery, reduced systemic side effects, prolonged action, and cost-effective treatment. These delivery systems consist of particle-based technologies like microparticles, nanoparticles, micelles liposomes, and protein nanoparticle.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients